Oxidation-redaction reactions. Electrode potential. Electrolysis
Oxidation and reduction. In the two preceding sections, we described precipitation reactions
(reactions producing a precipitate) and acid–base reactions (reactions involving
proton transfer). Here we discuss the third major class of reactions,
oxidation–reduction reactions, which are reactions involving a transfer of
electrons from one species to another.

As a simple example of an oxidation–reduction reaction, let us look
at what happens when you dip an iron nail into a blue solution of copper (II)
sulfate (figure 11.1). What you see is that the iron nail becomes coated with a
reddish-brown tinge of metallic copper. The molecular equation for this reaction
is
Fe(s) + CuSO4 (aq) → FeSO4
(aq) + Cu (s)
The net ionic equation is
Fe(s) + Cu2+ (aq) → Fe2+
(aq) + Cu(s)
The electron-transfer aspect of the reaction is apparent from this
equation. Note that each iron atom in the metal loses two electrons to form an
iron (II) ion, and each copper (II) ion gains two electrons to form a copper
atom in the metal. The net effect is that two electrons are transferred from
each iron atom in the metal to each copper (II) ion. The concept of oxidation
numbers was developed as a simple way of keeping track of electrons in a
reaction. Using oxidation numbers, you can determine whether electrons have been
transferred from one atom to another. If electrons have been transferred, an
oxidation–reduction reaction has occurred [15, p.185].
Oxidation numbers. We define the oxidation
number (or oxidation
state) of an atom in a substance as the actual charge of the atom if it exists as a
monatomic ion, or a hypothetical charge assigned to the atom in the substance by
simple rules. An oxidation–reduction reaction is one in which one or more atoms
change oxidation number, implying that there has been a transfer of
electrons. Consider the combustion of calcium metal in oxygen gas (figure
11.2).
2Ca(s) + O2 (g) →
2CaO(s)
This is an oxidation–reduction reaction. To see this, you assign
oxidation numbers to the atoms in the equation and then note that the atoms
change oxidation number during the reaction.
Since the oxidation number of an atom in an element is always zero,
Ca and O in O2 have oxidation numbers of zero. Another rule follows
from the definition of oxidation number: The oxidation number of an atom that
exists in a substance as a monatomic ion equals the charge on that ion. So the
oxidation number of Ca in CaO is +2 (the charge on Ca2+), and the
oxidation number of

Figure 11.2 The burning of calcium metal in oxygen.
The burning calcium emits a red-orange
flame.
O in CaO is -2 (the charge on O2-). To emphasize these
oxidation numbers in an equation, we will write them above the atomic symbols in
the formulas.
o
o
+2
-2
2Ca + O2 → 2CaO
From this, you see that the Ca and O atoms change in oxidation number
during the reaction. In effect, each calcium atom in the metal loses two
electrons to form Ca2+ ions, and each oxygen atom in O2
gains two electrons to form O2- ions. The net result is a transfer of
electrons from calcium to oxygen, so this reaction is an oxidation–reduction
reaction. In other words, an oxidation–reduction reaction (or redox reaction) is a reaction in which electrons are
transferred between species or in which atoms change oxidation
number.
Note that calcium has gained in oxidation number from 0 to +2. (Each
calcium atom loses two electrons.) We say that calcium has been oxidized. Oxygen, on the other hand,
has decreased in oxidation number from 0 to -2. Each oxygen atom gains two
electrons.
We say that oxygen has been reduced. An oxidation–reduction
reaction always involves both oxidation (the loss of electrons) and reduction
(the gain of electrons).Formerly, the term oxidation meant “reaction with
oxygen.” The current definition greatly enlarges the meaning of this term.
Consider the reaction of calcium metal with chlorine gas (figure 11.3); the
reaction looks similar to the burning of calcium in oxygen. The chemical
equation is
o o
+2
-2
Ca + Cl2 → CaCl2
In this reaction, the calcium atom is oxidized, because it increases in oxidation number (from 0 to +2, as in the previous equation). Chlorine is reduced; it decreases in oxidation number from 0 to -1. This is clearly an oxidation–reduction reaction that does not involve oxygen [15, p. 186].

Figure 11.3 The burning of calcium metal in chlorine. The reaction appears similar to the burning
of calcium in oxygen.
Oxidation-Number Rules. So far, we have used two rules for obtaining oxidation numbers: (1)
the oxidation number of an atom
in an element is zero, and (2) the oxidation number of an atom in a monatomic
ion equals the charge on the ion. These and several other rules for assigning
oxidation numbers are given in table 11.1.
In molecular substances, we use these rules to give the
approximate charges on the atoms. Consider the molecule SO2.
Oxygen atoms tend to attract electrons, pulling them from other atoms (sulfur in
the case of SO2). As a result, an oxygen atom in SO2 takes
on a negative charge relative to the sulfur atom. The magnitude of the charge on
an oxygen atom in a molecule is not a full -2 charge as in the O2-
ion. However, it is convenient to assign an oxidation number of -2 to oxygen in
SO2 (and in most other compounds of oxygen) to help us express the
approximate charge distribution in the molecule. Rule 3 in table 11.1 says that
an oxygen atom has an oxidation number of -2 in most of its compounds.
Rules 4 and 5 are similar in that they tell you what to expect for
the oxidation number of certain elements in their compounds. Rule 4, for
instance, says that hydrogen has an oxidation number of +1 in most of its
compounds.
Rule 6 states that the sum of the oxidation numbers of the atoms in a
compound is zero. This rule follows from the interpretation of oxidation numbers
as (hypothetical) charges on the atoms. Because any compound is electrically
neutral, the sum of the charges on its atoms must be zero. This rule is easily
extended to ions: the sum of the oxidation numbers (hypothetical charges) of the
atoms in a polyatomic ion equals the charge on the ion.
You can use Rule 6 to obtain the oxidation number of one atom in a
compound or ion, if you know the oxidation numbers of the other atoms in the
compound or ion. Consider the SO2 molecule. According to Rule
6,
(Oxidation number of S) +2 × (oxidation number of O) =
0
Or
(Oxidation number of S) +2 × (-2) = 0.
Therefore,
Oxidation number of S (in SO2) = -2 × (-2) =
+4
Table 11.1
Rules for Assigning Oxidation Numbers
|
Rule |
Applies
to |
Statement |
|
1 |
Elements |
The oxidation number of an atom in an element is
zero. |
|
2 |
Monatomic
ions |
The oxidation number of an atom in a monatomic ion equals the
charge on the ion. |
|
3 |
Oxygen |
The oxidation number of oxygen is -2 in most of its compounds.
(An exception is O in H2O2 and other peroxides,
where the oxidation number is -1.) |
|
4 |
Hydrogen |
The oxidation number of hydrogen is +1 in most of its
compounds. (The oxidation number of hydrogen is -1 in binary compounds
with a metal, such as CaH2.) |
|
5 |
Halogens |
The oxidation number of fluorine is -1 in all of its compounds.
Each of the other halogens (Cl, Br, I) has an oxidation number of -1 in
binary compounds, except when the other element is another halogen above
it in the periodic table or the other element is
oxygen. |
|
6 |
Compounds
and ions |
The sum of the oxidation numbers of the atoms in a compound is
zero. The sum of the oxidation numbers of the atoms in a polyatomic ion
equals the charge on the
ion. |
Describing Oxidation–Reduction Reactions. We use special terminology to describe oxidation–reduction reactions.
To illustrate this, we will look again at the reaction of iron with
copper (II) sulfate. The net ionic equation
is
Fe(s) +
Cu2+(aq) →
Fe2+(aq) + Cu(s)
We can write this reaction in terms of two half-reactions. A half-reaction is one of two parts of an oxidation–reduction
reaction, one part of which involves a loss of electrons (or increase of
oxidation number) and the other a gain of electrons (or decrease of oxidation
number). The half-reactions for the preceding equation are
Fe(s) → Fe2+(aq) + 2e- (electrons
lost by Fe)
Cu2+(aq) + 2e- → Cu(s) (electrons
gained by Cu2+)
Oxidation is the half-reaction in
which there is a loss of electrons by a species (or an increase of oxidation
number of an atom).
Reduction is the half-reaction in
which there is a gain of electrons by a species (or a decrease in the oxidation
number of an atom).
Thus, the equation Fe(s) → Fe2+(aq) + 2e- represents the
oxidation half-reaction, and
the equation Cu2+(aq) + 2e- → Cu(s)
represents the reduction half-reaction.
Recall that a species that is oxidized loses electrons (or contains
an atom that increases in oxidation number) and a species that is reduced gains electrons (or contains
an atom that decreases in oxidation number). An oxidizing agent is a species that oxidizes another species; it is
itself reduced. Similarly, a reducing agent is a species that
reduces another species; it is itself oxidized. In our example
reaction, the copper (II) ion is
the oxidizing agent, whereas iron metal is the reducing agent [15, p.
187].
Some Common Oxidation–Reduction Reactions. Many oxidation–reduction reactions can be described as one of the
following:
1.
Combination reaction
2.
Decomposition reaction
3.
Displacement reaction
4.
Combustion reaction
We will describe examples of each of these in this
section.
Combination Reactions A combination reaction is a reaction in which two substances combine
to form a third substance. Note that not all combination reactions are
oxidation–reduction reactions. However, the simplest cases are those in which
two elements react to form a
compound; these are clearly oxidation–reduction reactions.
2Na(s) + Cl2
(g) → 2NaCl(s)
Antimony and chlorine also combine in a fiery
reaction.
2Sb + 3Cl2 → 2SbCl3
Some combination reactions involve compounds as reactants and are not
oxidation–reduction reactions. For example,
CaO (s) + SO2 (g) →
CaSO3(s)
(If you check the oxidation numbers, you will see that this is not an
oxidation–reduction reaction.)
Decomposition Reactions A decomposition reaction is a reaction in which a single compound reacts
to give two or more substances. Often these reactions occur when the temperature is raised. In
Chapter 1, we described the decomposition of mercury (II) oxide into its elements when the
compound is heated. This is an
oxidation–reduction reaction.
2HgO(s) → 2Hg (l) + O2 (g)
Another example is the preparation of oxygen by heating potassium
chlorate with manganese(IV) oxide as a catalyst.
2KClO3(s) → 2KCl(s) + 3O2
(g)
MnO2
In this reaction, a compound decomposes into another compound and an
element; it also is an oxidation–reduction reaction.
Not all decomposition reactions are of the oxidation–reduction type.
For example, calcium carbonate at high temperatures decomposes into calcium
oxide and carbon dioxide.
CaCO3(s) → CaO (s) + CO2
(g)
Displacement Reactions. A displacement reaction (also called a
singlereplacement reaction) is
a reaction in which an element reacts
with a compound, displacing another element from it.
Since these reactions involve an element and one of its compounds, these must be
oxidation–reduction reactions. An example is the reaction that occurs when you dip
a copper metal strip into a solution of silver nitrate.
Cu(s) +
2AgNO3 (aq) →
Cu(NO3)2(aq) + 2Ag(s)
From the molecular equation, it appears that copper displaces silver
in silver nitrate, producing crystals of silver metal and a greenish-blue
solution of copper (II) nitrate. The net ionic equation, however, shows that the
reaction involves the transfer of electrons from copper metal to silver
ion:
Cu(s) + 2Ag+
(aq) → Cu2+
(aq) + 2Ag(s)
When you dip a zinc metal strip into an acid solution, bubbles of
hydrogen form on the metal and escape from the solution.
Zn(s) + 2HCl (aq) → ZnCl2 (aq) + H2 (g)
Zinc displaces hydrogen in the acid, producing zinc chloride solution
and hydrogen gas. The net ionic equation is
Zn(s) + 2H+
(aq) → Zn2+
(aq) + H2
(g)
Whether a reaction occurs between a given element and a monatomic ion
depends on the relative ease with which the two species gain or lose electrons.
Figure 11.4 shows the activity series of the elements, a listing of the elements
in decreasing order of their ease of losing electrons during reactions in
aqueous solution. The metals listed at the top are the strongest reducing agents
(they lose electrons easily); those at the bottom, the weakest. A free element
reacts with the monatomic ion of another element if the free element is above
the other element in the activity series. The highlighted elements react slowly
with liquid water, but readily with steam, to give
H2.
Consider this reaction:
2K(s) + 2H+
(aq) → 2K+
(aq) + H2
(g)
You would expect this reaction to proceed as written, because
potassium metal (K) is well above hydrogen in the activity series. In fact,
potassium metal reacts violently with water, which contains only a very small
percentage of H+ ions. Imagine the reaction of potassium metal with a
strong acid like HCl!
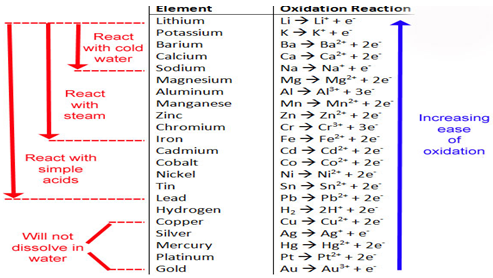
Figure 11.4 Activity Series of the Elements
Combustion Reactions.
A combustion reaction
is a reaction in which a
substance reacts with oxygen, usually with the rapid release of heat to produce
a flame. The products
include one or more oxides. Oxygen changes oxidation number from 0 to -2, so combustions are
oxidation–reduction reactions.
Organic compounds usually burn in oxygen or air to yield carbon
dioxide. If the compound contains hydrogen (as most do), water is also a
product. For instance, butane (C4H10) burns in air as
follows:
2C4H10 (g) + 13O2 (g) → 8CO2 (g) + 10H2O (g)
Many metals burn in air as well. Although chunks of iron do not burn
readily in air, iron wool, which consists of fine strands of iron, does. The
increased surface area of the metal in iron wool allows oxygen from air to react
quickly with it.
4Fe(s) + 3O2
(g) →
2Fe2O3(s)
[15, p. 190]
Balancing Simple Oxidation–Reduction Equations Oxidation–reduction reactions can often be quite difficult to
balance. Some are so complex in fact that chemists have written computer
programs to accomplish the task. In this section, we will develop a method for
balancing simple oxidation–reduction reactions that can later be generalized for
far more complex reactions.
One of the advantages of using this technique for even simple
reactions is that you focus on what makes oxidation–reduction reactions
different from other reaction types.
At first glance, the equation representing the reaction of zinc metal
with silver (I) ions in solution might appear to be
balanced.
Zn(s) + Ag+ (aq) → Zn2+
(aq) + Ag(s)
However, because a balanced chemical equation must have a charge
balance as well as a mass balance, this equation is not balanced: it has a total
charge of +1 for the reactants and +2 for the products. Let us apply the
half-reaction method for balancing this equation [15, p.
192].
Half-Reaction Method Applied to Simple Oxidation–Reduction Equations.
The half-reaction method
consists of first separating the equation into two half-reactions,
one for oxidation, the other for reduction. You balance each
half-reaction, then combine them to obtain a balanced oxidation–reduction
reaction. Here is an illustration of the process. First we identify the
species being oxidized and reduced and assign the appropriate oxidation
states.
Zn(s) + Ag+ (aq) → Zn2+ (aq) +
Ag(s)
Next, write the half-reactions in an unbalanced
form.
Zn → Zn2+ (oxidation)
Ag+ → Ag (reduction)
Next, balance the charge in each equation by adding electrons to the
more positive side to create balanced half-reactions. Following this procedure,
the balanced half-reactions are:
Zn → Zn2+ + 2e- (oxidation
half-reaction)
Ag+ + e- → Ag (reduction
half-reduction)
Note that the number of electrons that Zn loses during the oxidation
process (two) exceeds the number of electrons gained by Ag+ during
the reduction (one). Since, according to the reduction half-reaction, each
Ag+ is capable of gaining only one electron, we need to double the
amount of Ag+ in order for it to accept all of the electrons produced
by Zn during oxidation.
To meet this goal and obtain the balanced oxidation–reduction
reaction, we multiply each half-reaction by a factor (integer) so that when we
add them together, the electrons cancel.
We multiply the first equation by 1 (the number of electrons in the
second half-reaction) and multiply the second equation by 2 (the number of
electrons in the first half-reaction).
1 × (Zn → Zn2+ + 2e-)
The electrons cancel, which finally yields the balanced
oxidation–reduction equation:
Zn(s) + 2Ag+
(aq) → Zn2+
(aq) + 2Ag(s)
The majority of the chemical reactions discussed in this chapter take
place in solution. This is because the reaction between two solid reactants
often proceeds very slowly or not at all.
In a solid, the molecules or ions in a crystal tend to occupy
approximately fixed positions, so the chance of two molecules or ions coming
together to react is small. In liquid solutions, reactant molecules are free to
move throughout the liquid; therefore, reaction is much faster.
When you run reactions in liquid solutions, it is convenient to
dispense the amounts of reactants by measuring out volumes of reactant
solutions. In the next two sections, we will discuss calculations involved in
making up solutions [15, p. 193].
Oxidizing and Reducing Agents. Chemists frequently use the terms oxidizing agent and reducing agent to describe certain of
the reactants in redox reactions, as in statements like fluorine gas is a
powerful oxidizing agent, or calcium metal is a good reducing agent. Let us
briefly consider the meaning of these terms.
In a redox reaction, the substance that makes it possible for some
other substance to be oxidized is called the oxidizing agent, or oxidant. In doing so, the oxidizing
agent is itself reduced.
Similarly, the substance that causes some other substance to be
reduced is called the reducing
agent, or reductant. In
the reaction, the reducing agent is itself oxidized or stated in other ways
an oxidizing agent
(oxidant):
·
causes another substance to be oxidized
·
contains an element whose oxidation state decreases in a redox
reaction
·
gains electrons (electrons are found on the left side of its half-equation)
·
is reduced
·
a reducing agent (reductant)
·
causes another substance to be reduced
·
contains an element whose oxidation state increases in a redox
reaction
·
loses electrons (electrons are found on the right side of its
half-equation)
·
is oxidized
In general, a substance with an element in one of its highest
possible oxidation states is an oxidizing agent. If the element is in one of its
lowest possible oxidation states, the substance is a reducing
agent.
The oxidation state of the nitrogen in dinitrogen tetroxide is nearly
the maximum value attainable, and hence N2O4 is generally
(N2O4) an oxidizing agent. Conversely, the nitrogen atom
in hydrazine (N2H4) is in nearly the lowest oxidation
state, and hence hydrazine is generally a reducing agent. When these two liquid
compounds are mixed, a vigorous reaction takes place:
N2O4 (l) + 2N2H4 (l) →
3N2 (g) + 4H2O (g)
In this reaction, is the oxidizing agent and is the reducing agent.
This reaction releases so much energy that it is used in some rocket propulsion
systems.
Certain substances in which the oxidation state of an element is
between its highest and lowest possible values may act as oxidizing agents in
some instances and reducing agents in others. For example, in the reaction of
hydrazine with hydrogen to produce ammonia, hydrazine acts as an oxidizing
agent.
N2H4 (l) + H2 (g) → 2NH3
(g)
Permanganate ion, is a versatile oxidizing agent that has many uses
in the chemical laboratory. In the next section, we describe its use in the
quantitative analysis of iron that is, the determination of the exact
(quantitative) amount of iron in an iron-containing material. Ozone, a triatomic
form of oxygen, is an oxidizing agent used in water purification, as in the
oxidation of the organic compound phenol,
C6H5OH (aq) + 14O3 (g) → 6CO2
(g) + 3H2O (l) + 14O2 (g)
Aqueous sodium hypochlorite, NaOCl(aq), is a powerful oxidizing
agent. It is the active ingredient in many liquid chlorine bleaches. The
bleaching action of NaOCl(aq) is associated with the reduction of the
OCl- ion to Cl- ;the electrons required for the reduction
come from colored compounds in stains. The bleaching action of NaOCl(aq) is
demonstrated.
Thiosulfate ion, S2O32-, is an
important reducing agent. One of its industrial uses is as an antichlor to
destroy residual chlorine from the bleaching of fibers.
S2O32- (aq) + 4Cl2 (aq) +
5H2O (l) → 2HSO4- (aq) + 8H+ (aq) + 8Cl-
(aq)
Oxidizing and reducing agents also play important roles in biological
systems - in photosynthesis (using solar energy to synthesize glucose),
metabolism (oxidizing glucose), and the transport of oxygen [3, p.
199].
Electrolysis
(a)
Cells and electrolysis. In cells, oxidation-reduction reactions proceed spontaneously, and
the chemical energy accompanying the chemical reactions is converted to
electric energy. If voltage is applied to the cell from the direction
reverse to the electromotive force, a chemical reaction that corresponds to
the negative electromotive force is induced. In other words, reactions
that do not occur spontaneously are now induced by the electric energy.
This process is called electrolysis. The charging of a lead
storage battery is an example of electrolysis.
The total reaction of the Daniell cell is as
follows.
Zn + Cu2+ (aq) → Zn2+ (aq) +
Cu
Suppose a voltage higher than is applied in the direction reverse to
the electromotive force, the reverse reaction takes place. Thus, zinc will
deposit and copper will begin to dissolve.
Zn2+ (aq) + Cu → Zn + Cu2+
(aq)
Figure 11.5 shows a schematic representation of the chemical reaction
which occurs when a reverse voltage is applied to the Daniel cell.
(b)
Faraday’s law of electrolysis. In the first half of the 19th century, Faraday
investigated the relation between the quantity of electricity which flows
in a cell and the quantity of substances chemically changed at the electrodes
during the electrolysis. He summarized the results in two laws in
1833.
Faraday’s law of electrolysis
1.
The quantity of substances produced at the electrodes is proportional
to the quantity of electricity that flows in the cell.
2.
When a certain quantity of electricity flows in the cell, the number
of moles of substances changed at the electrodes is constant regardless of the
type of substance. For instance, the quantity of electricity necessary to
deposit 1 mole of a monovalent metal is 96,485 C (Coulomb) regardless of the
type of metal.
Faraday’s Laws of Electrolysis.
·
Mass of an element produced at an electrode is proportional to the
amount of electrical charge Q passed through the liquid. If a current of
I Amperes (A) is passed through an electrolyte solution for t
seconds (s), we have (1)
Q = It (1)
where the units of Q is the Coulomb (C). Obviously, C = A ×
s.
The mass of element produced is proportional to the equivalent weight
of the element.
·
The concept of “equivalent weights” is no longer recommended by
IUPAC. However, it remains a useful concept in this context. One way to think
about equivalent weight in the context of electrolysis is as the ratio of the
molar mass (M) of the substance to the number of moles of electrons n
that need to be added or removed to neutralize it. That is
(2),
E = M/n (2)
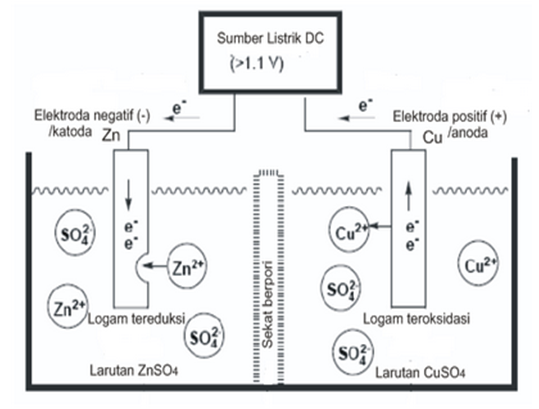
Figure 11.5 Electrolysis. A reaction reverse to that in the Daniell cell takes
place. Zinc deposits and copper dissolves [31, p. 15]
To illustrate, the equivalent weights E of a few elements
are:
Reaction;
M
(g mol–1); n;
E (g equiv–1)
H+(aq) + e- →
½H2(g)
1.008
1
1.008
Fe2+(aq) + 2e- →
Fe(s)
55.847
2
27.924
Fe3+(aq) + 3e- →
Fe(s)
55.847
3
18.616
Cl-(aq) → ½Cl2(g) +
e-
35.453
1
35.453
The two laws can be combined into a convenient form by introducing
the quantity known as the Faraday (F), which is the charge carried by one
mole of electrons:
F = 1.6022 × 10–19 C × 6.0220 × 1023 mol–1 = 96,485 C
mol–1.
Since one mole of electrons corresponds to one equivalent of the
element, we may also think of the units of the F as C equiv–1
[31, p.16].
(c)
Industrially important electrolyses. The 1st electrolysis attempted was the hydrolysis of water (1800).
Davy immediately followed and successfully isolated alkali and alkaline
earth metals. Even now electrolysis is used to produce various metals.
Electrolysis is particularly useful for the production of metals with high
ionization tendencies (e.g.,
aluminum).
The industrial production of aluminum by electrolysis was achieved in
1886 independently by the American inventor Charles Martin Hall (1863-1914) and
the French inventor Paul Louis Toussaint Héroult (1863-1914) at the same time.
The success of this electrolysis was due to the use of molten
Na3AlF6 as the solvent of the ore (aluminum oxide; alumina
Al2O3).
As a requirement for successful electrolysis, ions can migrate to the
electrodes. An obvious way to give mobility to ions is to use its aqueous
solution. However, in the case of electrolysis of alumina, an aqueous solution
is inadequate because water is more readily reduced than the aluminum ion as
shown below.
Al3+ + 3e- → Al normal electrode
potential = -1.662 V
2H2O +2e- → H2 + 2OH-
normal electrode potential = -0.828 V
Another method would be the use of molten salt. The trouble is that
the melting point of Al2O3 is as high as 2050 ºC, and
electrolysis at such a high temperature is not realistic. However, the melting
point of a mixture of Al2O3 and
Na3AlF6 is ca. 1000 ºC, and this temperature is
easy to attain.
The details of the procedure are as follows: the ore, bauxite,
contains various metal oxides as impurities. The ore is treated with alkali, and
only amphoteric aluminum oxide dissolves. Insoluble materials are filtered off,
and carbon dioxide is blown through the filtrate to cause hydrolysis (of salts).
Alumina is deposited.
Al2O3(s) + 2OH-(aq) →
2AlO2- (aq) + H2O(l)
2CO2 + 2AlO2-(aq) +
(n+1)H2O(l) → 2HCO3- (aq) +
Al2O3 × nH2O(s)
The alumina thus obtained is mixed with Na3AlF6 and then molten
salt electrolysis of the mixture is carried out. The reactions in the
electrolytic cell are complicated. It is likely that initially alumina reacts
with Na3AlF6 and then electrolytic reactions take
place.
Al2O3 + 4AlF63- →
3Al2OF62- +
6F-
The electrode
reactions are as follows.
Negative electrode: 2Al2OF62- +
12F- + C → 4AlF63- + CO2 +
4e-
Positive electrode: AlF63- + 3e- →
Al + 6F-
Total reaction: 2Al2O3 + 3C → 4Al +
3CO2-
The purity of aluminum obtained by this procedure is ca. 99.55
%. Aluminum is used as such and as alloys with other metals. The properties are
excellent and, in addition, the price is modest. However, it must be remembered
that the production of aluminum requires a tremendous amount of electricity [31,
p. 17].