Complex compounds
Coordination compound. You have come across compounds like Na[Ag(CN)2] and
Na2[Zn(CN)4]. Such compounds are referred to as
coordination compounds or complex compounds. Coordination compounds play an
important role in the chemical industry and in life itself. For example, the
Ziegler- Natta catalyst which is used for polymerization of ethylene, is a
complex containing the metals aluminum and titanium. Metal complexes play
important role in biological systems. For example, chlorophyll, which is vital
for photosynthesis in plants, is a magnesium complex and hemoglobin, which
carries oxygen to animal cells, is an iron complex. These are the compounds that
contain a central atom or ion, usually a metal, surrounded by a number of ions
or molecules. The complexes tend to retain their identity even in solution,
although partial dissociation may occur. Complex ion may be cationic, anionic or
nonionic, depending on the sum of the charges of the central atom and the
surrounding ions and molecules [29, p. 1].
Werner’s Coordination Theory. Coordination compounds were known in eighteenth century. It was a
mystery for the chemist, of those days to understand as to why a stable salt like
CoCl3 reacts with varying number of stable molecules or compounds such as ammonia to give
several new compounds: CoCl3 × 6NH3, CoCl3 ×
5NH3 and CoCl3 × 4NH3; and what are their
structures? These compounds differed from each other in their chloride ion
reactivity. Conductivity measurements on solutions of these compounds showed
that the number of ions present in solution for each compound are different.
Several theories were proposed, but none could satisfactorily explain all the
observable properties of these compounds and similar other series of compounds
which had been prepared by then. It was only in 1893 that Werner put forward a
set of ideas which are known as Werner’s coordination theory, to explain the
nature of bonding in complexes. His theory has been a guiding principle in
inorganic chemistry and in the concept of valence. The important postulates of
Werner’s theory are:
1.
Metals exhibit two types of valence:
a)
Primary valence
(ionizable)
b)
Secondary valence (non-ionizable).
Primary or ionizable valence is satisfied by negative ions and
corresponds to oxidation state of the metal. The secondary or non-ionizable
valence, which is satisfied by negative, positive or neutral groups, is equal to
the coordination number of metal ion.
Every metal tends to satisfy both its primary and secondary
valence.
2.
The secondary valence is directed toward fixed positions in space
i.e. this has spatial arrangement corresponding to different coordination
number.
For the complexes CoCl3 × 6NH3,
CoCl3 × 5NH3 and CoCl3 × 4NH3, the
number of ionizable ions in these complexes are three, two and one,
respectively. It has been proved by precipitation reactions and conductivity
measurements. On the basis of Werner’s postulate these compounds are formulated
as:
[Co(NH3)6]Cl3,
[Co(NH3)5Cl]Cl2 and
[Co(NH3)4Cl2]Cl, respectively, the species
inside the square brackets being the complex ion and outside the square brackets
the ionisable ions.
One of the three chloride ions satisfy both primary and secondary
valence. He also postulated that octahedral, tetrahedral and square planar
shapes are more common for coordination compounds of transition elements. Six
coordinated complexes such as [Ni(NH3)6]2+ and
[Co(NH3)6]3+ are octahedral whereas four
coordinated such as [NiCl4]2- and
[Ni(CN)4]2- are tetrahedral and square planar,
respectively [29, p. 2].
Classification of complex compounds. Currently, there are several
classifications of complex compounds.
Classification on the nature of the
ligands. The basis
of this classification is the nature of the ligands of complexing agent:
·
Acidocomplexes (from latin acidum - acid) ligands are the residues of
acids: CN-, Cl-, Br-, I-, for example H[AuCl4],
K2[HgI4], the residues of many organic acids: oxalate ion
C2O42-, aminopolycarbonic acid residues, etc.;
·
Amminecomplexes. The ligands are molecules of ammonia NH3,
for example, [Cu (NH3)4](NO3)2, [Ag
(NH3)2]Cl;
·
Aquacomplexes. The ligands are water molecules:
[Cr(H2O)6]Cl3,
[Cu(H2O)4](NO3)2;
·
Hydroxocomplexes. The ligands are hydroxide ions:
K3[Co(OH)6];
·
Carbonyl
complexes. The ligands are CO molecules: [Ni(CO)4],
[Cr(CO)6].
The inner sphere may be acid
residues and neutral groups, for example: [Cr(NH3)4Cl2]Cl. Such
complexes are mixed.
Chelation-complexes are complexes in which the metal atom-complexing
agent is associated with organic ligands in several relationships (see table
10.1). The complexing agent and ligands form loops, the strength of which is
change due to Chugaev’s rule (most stable of these compounds contain in the
inner sphere of five- or six-membered rings).
Table 10.1
The most common ligands:
|
Types of
ligand |
Chemical
formula |
The name of the
ligand |
|
Negative ions |
CH3COO- |
acetato- |
|
F-, Cl-,Br-, I- |
the fluoro
-, chloro- bromo-, iodo- | |
|
OH- |
hydroxo- | |
|
CN- |
cyano- | |
|
SCN- |
thiocyano, tiocianato- | |
|
NO2- |
nitro- | |
|
NO3- |
nitrato- | |
|
SO42- |
sulphato- | |
|
SO32- |
sulphito- | |
|
S2- |
thio-, sulphido- | |
|
S22- |
disulphido- | |
|
O22- |
peroxo- | |
|
Positive ions |
NH4+ |
ammonium- |
|
OH3+ |
hydroxoniy- | |
|
Neutral molecules |
NH3 |
ammine- |
|
CO |
carbonyl- | |
|
H2O |
aqua- | |
|
NO |
nitrosyl- | |
|
N(CH3)3 |
trimethylamine- | |
|
Chelateformation particles |
C2O42- |
oxalate- |
|
NH2-CH2-COO- |
glicinato- | |
|
NH2-CH2-CH2-NH2 |
ethylenediamine- |
The ligands are characterized by denticity - ability to form multiple
coordination bonds with ions-complexing agent. There are monodentate and
polydentate ligands. The coordination number is equal to the number of
monodentate ligands coordinating by the central atom. For example,
[Cu(NH3)4]2+, the coordination number is 4. Due
to NH3 (ammonia) is monodentate ligand, and 4 molecules of ammonia
are filled 4 places around central atom (Cu2+) [30, p.
15].
The
nomenclature of complex compounds. The name of the complex compounds
are composed as follows:
Name the ligands
first, in alphabetical order, then the central atom or ion. Name of the complex
is written in one word. Neutral ligands called without changes; in the names of
negatively charged ligands add «o» to the end. Greek prefixes are used to
designate the number of each type of ligand in the complex ion, e.g. di-, tri-
and tetra-
Name of
complexing agent depends on the charge of the complex. For neutral and cationic
– English name of the cation. For the anionic complex - the Latin suffix «ate».
Indicate the degree of oxidation of complexing agent using Roman numerals in
parentheses.
Examples of
names of the ligand: Aqua - H2O, NH3 - ammine,CO – carbon
monoxide, NO - nitrosyl, OH- - hydroxo, CN- - cyano,
NO2-, SO32-nitro-, carbonato-,
sulphito-, SO42- - sulphato- , Cl- -chloro-.
For Example:
[Ag
(NH3)2]Cl Diamminesilver(I) chloride;
[Cu(NH3)4](OH)2
Tetraamminecopper(II) hydroxide;
[Al(H2O)5OH]Cl2
Hydroxopentaaqaaluminium(III) chloride;
[Pt(H2O)3OH]NO3
Hydroxotriaquaplatinum(II) nitrate;
[Co(NH3)4CO3]Cl
Carbonatotetramminecobalt(III) chloride;
[Al(H2O)6]Cl3 Hexaaquaaluminium(III)
chloride.
K3[Fe (CN)6] potassium
hexacyanoferrate(III);
[Cr(H2O)3F3]-trifluorotriaquachromium(III)
[30, p. 17]
Isomerism of
the complex compounds. Isomerism is a phenomenon, when substances have the same qualitative
and quantitative composition, but have a different structure, and hence
different properties.
There are
geometric, optical, hydrated, ionization, coordination, etc. types of isomerism
of complex compounds.
Geometric
(spatial) isomerism is common for complex compounds with different
(heterogeneous) ligands. The geometric isomers have different placement of
inhomogeneous ligands in a complex which has square-planar or octahedral
structure.
For example, for
the complex [Pt(NH3)2Cl2] with square-planar
structure, there are two geometric isomers (CIS-and TRANS-isomers) (see figure
10. 1), which explains the difference of their properties (different color,
dipole moment, reactivity):
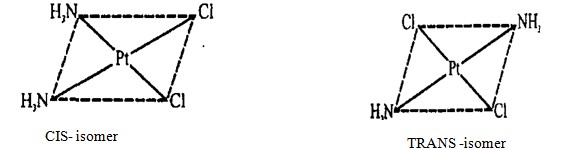
Figure 10.1 CIS-andTRANS-isomers
CIS (cis) refers to one side, close; and TRANS - (trans) - on different sides. For complex [Co(NH3)4Cl2] is octahedral structure (see figure 10.2) of geometrical isomers can be shown schematically
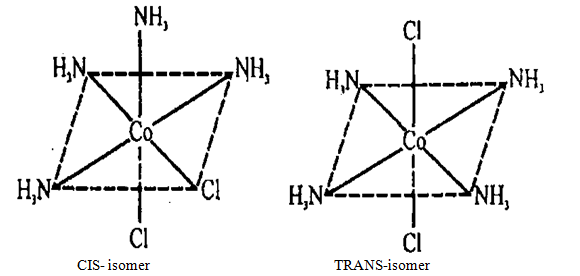
Figure 10.2 Complex [Co(NH3)4Cl2] is
octahedral structure
Hydrate isomers are substances which have the same composition, but
is different allocation of molecules of the solvent between the internal and
external spheres of complex compounds. For example, crystalline hydrate
corresponds to four CrCl3 × 6H2O
isomer:
[Cr(H2O)6]Cl3;
[Cr(H2O)5Cl]Cl2 ×
H2O;
[Cr(H2O)4Cl2]Cl ×
2H2O;
[Cr(H2O)3Cl3] ×
3H2O.
All isomers have different colour.
So:
[Cr(H2O)6]Cl3 -
purple;
[Cr(H2O)5Cl]Cl2 × H2O -
green;
[Cr(H2O)4Cl2]Cl × 2H2O -
dark green.
Ionization isomers are substances which have the same composition,
but isdetermined by the different distribution of charged ligands between
internal and external spheres of the complex. These isomers are also
distinguished by colour:
[CoBr(NH3)5]SO4 -
red-purple
[CoBr(NH3)5]SO4 →
[CoBr(NH3)5]2+ +
SO42-.
[CoSO4(NH3)6]Br -
red
[CoSO4(NH3)6]Br →
[CoSO4(NH3)6]+ +
Br-
Coordination
isomerism. When both positive and negative ions of a salt are complex ions and
the two isomers differ in the distribution of ligands between the cation and the
anion occurs coordination isomerism. For example
[Co(NH3)6][Cr(CN)6] and
[Cr(NH3)6][Co(CN)6],
[Pt(NH3)4][PtCl4] and
[Pt(NH3)3Cl][Pt(NH3)Cl3]
That is, this
type of isomerism is possible for compounds composed of two or more systems, and
complexing agnt exchange their ligands [30, p. 18].
Properties of
coordination compounds. Complex compounds are involved in various chemical
reactions-substitution, exchange, isomerization, redox processes.
1.
Stability
of the complex compounds in solutions.
Neutral
complexes (coordination compounds with no external coordination sphere) are
non-electrolytes, i.e. does not dissociate in the aqueous solutions on ions.
Complex
compounds with complex ion (cation or anion) dissociate in aqueous solutions on
Internal and external coordination spheres. This dissociation of complex
compounds is called the primary. It is almost completely. There are complex
compounds that have external coordination sphere, dissociate in aqueous
solutions as strong electrolytes. For example,
[Cu(NH3)4]SO4, K[AuCl4] such as
simple salt dissociation:
[Cu(NH3)4]SO4 →
[Cu(NH3)4]2+ +
SO42-
K[AuCl4] → K+ +
[AuCl4]-
In turn complex
ion is also capable to dissociate as electrolyte, but weak or middle force, that
is, reversible (secondary dissociation):
[Cu(NH3)4]2+ ⇄
Cu2+ + 4NH3
[AuCl4]- ⇄
Au3+ + 4Cl-
The stability
constant characterizes the equilibrium process of formation of a complex that
also happens speed. For example, the formation of the complex of
[Cu(NH3)4]2+, you can show the following
equations:
I-st step
Cu2+ + NH3 ⇄
[Cu(NH3)]2+;
II-nd step
[Cu(NH3)]2+ + NH3 ⇄
[Cu(NH3)2]2+;
III-d step
[Cu(NH3)2]2+ + NH3 ⇄
[Cu(NH3)3]2+;
IV-th step
[Cu(NH3)3]2+ + NH3 ⇄
[Cu(NH3)4]2+.
Each stage
corresponds to a certain value of the stability constant (or β).The General equation of complex
formation is
[Cu(NH3)4]2+ ⇄Cu2+ + 4NH3 ⇄
[Cu(NH3)4]2+
In the reaction
of complex compound [Ag(NH3)2]Cl, ions of hydrogen of
nitric acid react with ammonia molecules to produce a strong ammonium ion, which
can show the equation:
[Ag
(NH3)2]Cl + 2HNO3 → AgCl ↓ + 2 [NH4]
NO3
(NH4+ is usuall simplistic designation a
complex ion of ammonium).
As noted above, complex compounds participate in various chemical
reactions, such as substitution, exchange, isomerization, ox/red processes. Here
are some examples:
2.
Reactions
of substitution of ligands in a complex ion internal coordination sphere:
[Cu(H2O)4]SO4 + 4NH3 →
[Cu(NH3)4] SO4 +
4H2O;
Zn + 2Na[Au(CN)2] → 2Au + Na2
[Zn(CN)4]
3.
Exchange
reactions. Exchange reactions of complex compounds often find application in
analytical chemistry for the qualitative detection of certain ions.
For example,
detection of the Zn2+ cation with hexacyanoferrate (II) potassium
(yellow blood salt). As a result of the reaction a white sediment
hexacyanoferrate (II) potassium zinc formation can be shown by equation:
3ZnCl2
+ 2K4[Fe(CN)6] → К2Zn3[Fe(CN)6]2↓ +
6KCl
3Zn2+
+ 2К+ +
2[Fe(CN)6]4- → К2Zn3[Fe(CN)6]2↓
Reactions of
Turnbule blue and the Prussian blue formation is used for detection of the the
cations Fe2+ and Fe3+, respectively. It is proved that
Prussian blue and Turnbule blue are identical in composition, as a result of the
reaction they appear as dark blue precipitation:
FeCl2
+ К3[Fe(CN)6]→ КFe[Fe(CN)6]↓ + 2KCl;
Potassium
hexacyanoferrate(ІІІ)Turnbule blue
КFe[Fe(CN)6] ⇄К[FeFe(CN)6];
potassiumhexacyanoferrate(ІІ, ІІІ)
Fe2++
[Fe(CN)6]3- → КFe[Fe(CN)6]↓ ⇄
К[FeFe(CN)6]↓.
FeCl3
+ К4[Fe(CN)6] → КFe[Fe(CN)6]↓ + 3KCl;
Potassium
hexacyanoferrate (ІІ) Prussian
blue
КFe[Fe(CN)6] ⇄
К[FeFe(CN)6];
potassiumhexacyanoferrate(ІІ, ІІІ)
Fe3++
[Fe(CN)6]4- → КFe[Fe(CN)6]↓ ⇄ К[FeFe(CN)6]↓.
4.
Redox reactions:
2K4[Fe(CN)6] + Cl2 →
2K3[Fe(CN)6] + 2KCl;
4[Co(NH3)6]2+ + O2 +
2H2O → 4[Co(NH3)6]3+ + 4OH-
[30, p.20]