Theory of electrolytic dissociation
Theory of electrolytic conduction. Arrhenius theory of electrolytic conductance is also known as
Arrhenius theory of ionization since electrolytic dissociation into ions is
considered here.
Postulates of Arrhenius theory In aqueous solution, the molecules of an electrolyte undergo
spontaneous dissociation to form positive and negative ions.
The assumption made was that when an acid, base or salt is dissolved
in water, a considerable portion becomes spontaneously dissociated into positive
and negative ions. The Arrhenius assumption was based on degree of ionization,
so it valid on weak electrolytes (incomplete ionization) but it failure with
respect to strong electrolytes (complete ionization in either strong and weak
electrolytes).
The decreasing of equivalent conductance at high concentration as
Arrhenius suggest belong to decreasing of degree of ionization of electrolyte.
The Arrhenius interpretation concentrate about number of ions and he ignore the
mobility of ions. The Arrhenius suggestion are only apply on weak electrolytes
because the strong electrolytes are completely dissociate at high concentration
[27, p. 1].
Postulates of Arrhenius Theory:
1.
When dissolved in water, neutral electrolyte molecules are
split up into two types of charged particles.
These particles were called ions and the process was
termed ionization. The positively charged particles were
called cations and those having negative charge were
called anions.
The theory assumes that the ions are already present in the solid
electrolyte and these are held together by electrostatic force. When placed in
water, these neutral molecules dissociate to form separate
anions and cations.
A+ B- → A+ +
B-
For that reason, this theory may be referred to as the theory of
electrolytic dissociations.
2.
The ions present in solution constantly reunite to form neutral
molecules. Thus there is a state of equilibrium between the undissociated
molecules and the ions.
AB ↔ A+ + B-
Applying the Law of Mass Action to the ionic equilibrium we
have,
[ A + ][ B - ] / [AB] = K
(1)
where K is called the Dissociation constant (1).
3.
The charged ions are free to move through the solution to the
oppositely charged electrode. This is called as migration of ions. This movement
of the ions constitutes the electric current through electrolytes. This explains
the conductivity of electrolytes as well as the phenomenon of
electrolysis.
4.
The electrical conductivity of an electrolyte solution depends on the
number of ions present in solution. Thus the degree of dissociation of an
electrolyte determines whether it is a strong electrolyte or a weak
electrolyte.
We know that electrolytes dissociate in solution to form positive
ions (cations) and negative ions (anions).
AgNO3 → Ag+ +
NO3-
CuSO4 → Cu2+ +
SO42-
H2SO4 → 2H+ +
SO42-
As the current is passed between the electrode of the electrolytic
cell, the ions migrate to the opposite electrodes. Thus in the electrolytic
solution of AgNO3, the cations (Ag+) will move to the
cathode and anions (NO3- ) will move to the anode.
Usually different ions move with different rates. The migration of ions through
the electrolytic solution can be demonstrated by the following experiments
(figure 9.1).
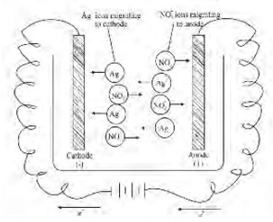
Figure 9.1 Nitration of ions through electrolytic solution to
opposite electrodes
5.
The properties of solution of electrolytes are the properties of
ions. The solution of electrolyte as a whole is electrically neutral unless an
electric field is applied to the electrodes dipped into it. Presence of hydrogen
ions (H+) renders the solution acidic while presence of hydroxide
ions (OH-) renders the solution basic.
6.
There are two types of electrolytes. Strong electrolytes are those
when dissolved in water are completely dissociated (ionized) into ions of
positive and negative charges. The total number of cations and anions produced
are equal to those in the formula of the electrolyte.
Al2(SO4)3 →
2Al3+ + 3SO42-
NaCl, KCl, AgNO3 etc., are few examples of strong
electrolytes.
In the case of weak electrolytes, there is partial dissociation into
ions in water and an equilibrium exists between the dissociated ions and the
undissociated electrolyte.
CH3COOH ↔ CH3COO- +
H+ Acetic acid is a weak
electrolyte in water and unionized acetic acid molecules are in
equilibrium with the acetate anions and H+ ions in
solution.
Evidences of Arrhenius theory of electrolytic
dissociation
1.
The enthalpy of neutralization of strong acid by strong base is a
constant value and is equal to -57.32 kJ. gm. equiv -1. This
aspect is well explained by adopting Arrhenius theory of electrolytic
dissociation. Strong acids and strong bases are completely ionized in water and
produce H+ and OH- ions respectively along with
the counter ions. The net reaction in the acid-base neutralization is the
formation of water from H+ and
OH-ions.
H+ + OH- →
H2O, DHro = -57.32
kJ.mol -1
2.
The colour of certain salts or their solution is due to the ions
present. For example, copper sulphate is blue due to Cu2+ ions.
Nickel salts are green due to Ni2+ ions. Metallic chromates are
yellow due to CrO42- ions.
3.
Ostwalds dilution law, common ion effect and solubility product and
other such concepts are based on Arrhenius theory.
4.
Chemical reactions between electrolytes are almost ionic reactions.
This is because these are essentially the reaction between oppositely charged
ions. For example,
Ag+ + Cl- →
AgCl↓
5.
Electrolytic solutions conduct current due to the presence of ions
which migrate in the presence of electric field.
6.
Colligative properties depend on the number of particles present in
the solution. Electrolytic solution has abnormal colligative properties. For
example, 0.1 molal solution of NaCl has elevation of boiling point about twice
that of 0.1 molal solution of non-electrolyte. The abnormal colligative
properties of electrolytic solutions can be explained with theory of
electrolytic dissociation.
Ostwald's dilution law for weak electrolytes. According to Arrhenius theory, weak electrolytes partially
dissociate into ions in water which are in equilibrium with the undissociated
electrolyte molecules. Ostwald's dilution law relates the dissociation constant
of the weak electrolyte with the degree of dissociation and the concentration of
the weak electrolyte. Consider the dissociation equilibrium of
CH3COOH which is a weak electrolyte in water.
CH3COOH ↔ CH3COO- +
H+
Ka = [
H + ][CH 3COO - ] /
[CH3COOH]
a is the degree of dissociation which represents the fraction of
total concentration of CH3COOH that exists in the completely
ionized state. Hence (1 - a) is the fraction of the total concentration of
CH3COOH, that exists in the unionized state. If 'C' is the total
concentration of CH 3COOH initially, then at equilibrium Ca,
Ca and C (1 - a) represent the concentration of H+,
CH3COO- and CH3COOH
respectively.
Then Ka = (Ca .C a) / C (1-a)
/ a2 C / (1-a)
If a is too small,
then Ka = a2C
a = root(Ka/C) also [H+] =
[CH3COO-] = Ca
[H+] = root (Ka.C)
Ka= a2C / (1-a) is known as the Ostwalds
dilution law. For weak bases,
Kb= a2C / (1-a) and a = rt
(Kb/C) at a = small
values.
Kb = dissociation constant for weak base.
This law fails for strong electrolytes. For strong
electrolytes, a tends to 1.0 and therefore
Ka increases tremendously [26, p. 1].
Strong and Weak Electrolytes. Solutes giving conducting solution in a suitable solvent are called
electrolytes. On the basis of degree of ionization, these electrolytes
have been divided into two categories.
a)
Strong
electrolytes
b)
Weak
electrolytes
Strong Electrolytes. Substances, which are highly dissociated and give solutions with high
conductance in water, are called strong electrolytes. Due
to the high degree of dissociation of strong electrolytes these substances
are good conductor of electricity i.e., aqueous solutions of these
substances have high value of molar conductance and on dilution
the increase in their molar conductance is very small. This is due to the
fact that such electrolytes are completely ionized at all dilutions
therefore on further dilution the number of current carrying
particles does not increase in the solution. Thus, solutions of
electrolytes that have high molar conductance, and increases very
slowly on dilution has a high degree of dissociation is called
strong electrolyte.
During the passage of an electric current through solutions, flow of
electricity is associated with the movement of particles, which are called
ions. The ions carrying positive charges and moving in the direction of
the current, i.e., towards the cathode, are referred to as cations and
those carrying a negative charge and moving in the opposite direction, i.e.,
towards the anode, are called anions [28, p.
2].
Weak Electrolytes. Weak acids and weak bases, e.g., amines, phenols, most carboxylic
acids and some inorganic acids and bases, such as hydrocyanic acid
and ammonia, and a few salts, e.g., mercuric chloride and cyanide,
are dissociated only to a small extent at reasonable concentration; this group
of compounds in general are called as weak
electrolytes.
The molar conductance of the solutions of these electrolytes
increases rapidly on dilution. The reason of this is that more molecules ionize
on dilution inspite of this they are never completely ionized. For these
electrolytes, the nature of the solvent is also important; a particular compound
may be strong electrolyte, being dissociated to large extent, in one solvent,
but may behave as weak electrolyte in other solvent due to low degree of
dissociation [28, p. 3].