Hydrolysis of salt
Hydrolysis. The reaction of an anion or cation with water accompanied by cleavage
of O–H bond is called hydrolysis. The term hydrolysis is derived from hydro, meaning water, and
lysis, meaning breaking. It may be noted that in anionic hydrolysis shown in the
solution becomes slightly basic (pH > 7) due to the generation of excess
OH– ions. In cationic
hydrolysis there is excess of H+ ions which makes the solution
slightly acidic (pH < 7) [24, p. 1].
Bronsted-Lowry concept of hydrolysis. HA and A– are conjugate acid-base pair
HA + H2O ↔ H3O+ + A–
weak
acid conjugate
base
Since HA is a weak acid (poor proton donor), its conjugate base, A–,
must be relatively strong (good proton acceptor). Owing to this fact,
A– ions tend to react with water by accepting proton from the latter
to form HA molecule (anionic hydrolysis),
A- + H2O → HA + OH−
The presence of OH– ions makes the solution
basic.
Similarly, BOH and B+ are a conjugate acid-base pair.
Since BOH is a weak base, its conjugate acid, B+, would be relatively
strong. Thus B+ would accept OH– ions from water to form
BOH molecules.
B+ + H2O → BOH + H+
The presence of excess H+ ions makes the solution acidic
[24, p. 2].
Hydrolysis of
salts. Hydrolysis
of salts disturbs the ionic equilibrium of water that causes change in
concentration of hydrogen and hydroxyl ions and leads to acidic or alkaline
reaction of a solution.
As it is known,
water is a weak electrolyte, ionizing slightly and
reversibly
HOH ↔ H+ + OH-
If water is absolutely pure, the concentrations of hydrogen and
hydroxyl ions are equal to each other and equal to 10-7 mole/l at
temperature of 25 °C. The product of these concentrations is named the ion
product for water Kw
Kw = C(H+) ∙ C(OH-) =
10-14
To determine the acidity-alkalinity of a medium the hydrogen
ion index pH and the hydroxyl ion index pOH are
used
pH= - lg
C(H+)
pOH = - lg C(OH-)
It is easy to
deduce from the above equations:
·
neutral
solution pH = 7;
·
acidic
solution pH < 7 (the higher acidity, the smaller it is);
·
alkaline
solution pH > 7 (the higher alkalinity, the larger it is).
It is obvious
that pH + pOH=14
There are
various methods of measuring pH values. Qualitatively the reaction of a solution
can be determined by means of special reagents called indicators, which change
colour depending on concentration of hydrogen ion.
The most widely
used indicators are litmus, phenolphthalein and methyl orange (table
8.1).
Table
8.1
Colours of
indicators
|
Indicator |
Reaction of
solution | ||
|
acid |
neutral |
alkaline | |
|
Methyl orange |
red |
orange |
yellow |
|
Phenolphthalein |
colourless |
pale crimson |
crimson |
|
Litmus |
red |
violet |
blue |
Any salt can be
obtained by neutralization of acids by bases. It is natural to assume,
therefore, that solutions, at least those witch are products of complete
displacement of hydrogen in acids by metals, must react neutral. However, this
assumption holds only for salts of strong acids and strong bases. Salts of weak
acids and strong bases, or vice versa, of strong acids and weak bases, do not
react neutral when dissolved in water. As an example, a solution of zinc
chloride ZnCl2 reacts acid, indicating an excess of hydrogen ions as
compared to hydroxyl ions C(H+) > C(OH-).
In contrast to
the previous example, a solution of potassium fluoride KF reacts alkaline which
is characteristic of hydroxyl ion and the ratio of hydrogen and hydroxyl ions is
C(H+) < C(OH-).
The ionization
theory attributes this phenomena to the interaction of water ions with the ions
of dissolved salts, resulting the formation of an excess of hydrogen or hydroxyl
ions. Although the concentration of hydrogen or hydroxyl ions is very low in
water, they are in equilibrium with an immense number of unionized water
molecules. When one of them is bound by the ions of the salt, the equilibrium is
disturbed, causing new water molecules to ionize, which may lead to accumulation
of considerable quantities of the other ion, making the solution react acid or
alkaline.
Any
reaction between the ions of a salt and the ions of water, attended with a
change in the concentration of the latter, is called hydrolysis of the salt.
The chief cause
of hydrolysis is the formation of slightly ionized substances (ions or
molecules) [25, p. 3].
Acid-Base Neutralization.
A solution is neutral when it contains equal concentrations of
hydronium and hydroxide ions. When we mix solutions of an acid and a base, an
acid-base neutralization reaction occurs. However, even if we mix
stoichiometrically equivalent quantities, we may find that the resulting
solution is not neutral. It could contain either an excess of hydronium ions or
an excess of hydroxide ions because the nature of the salt formed determines
whether the solution is acidic, neutral, or basic. The following four situations
illustrate how solutions with various pH values can arise following a
neutralization reaction using stoichiometrically equivalent
quantities:
1.
A strong acid and a strong base, such as HCl(aq) and NaOH(aq) will react to form a neutral solution since the conjugate partners
produced are of negligible strength (see figure 8.1):
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
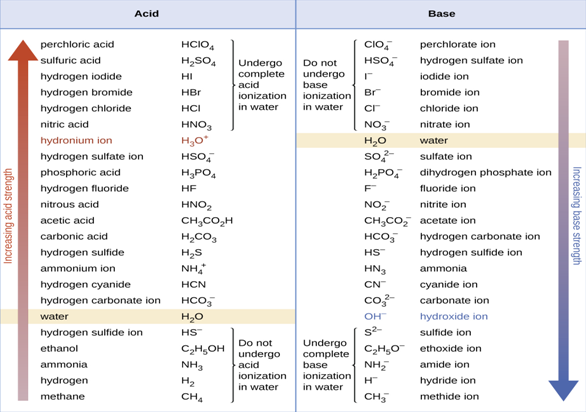
Figure 8.1 The chart shows the relative strengths of conjugate acid-base
pairs.
2.
A strong acid and a weak base yield a weakly acidic solution, not
because of the strong acid involved, but because of the conjugate acid of the
weak base.
3.
A weak acid and a strong base yield a weakly basic solution. A
solution of a weak acid reacts with a solution of a strong base to form the
conjugate base of the weak acid and the conjugate acid of the strong base. The
conjugate acid of the strong base is a weaker acid than water and has no effect
on the acidity of the resulting solution. However, the conjugate base of the
weak acid is a weak base and ionizes slightly in water. This increases the
amount of hydroxide ion in the solution produced in the reaction and renders it
slightly basic.
4.
A weak acid plus a weak base can yield either an acidic, basic, or
neutral solution. This is the most complex of the four types of reactions. When
the conjugate acid and the conjugate base are of unequal strengths, the solution
can be either acidic or basic, depending on the relative strengths of the two
conjugates. Occasionally the weak acid and the weak base will have the
same strength, so their respective conjugate base and acid will have the
same strength, and the solution will be neutral. To predict whether a particular
combination will be acidic, basic or neutral, tabulated K values of the conjugates must be compared [2, p. 804].
Salts of Weak Bases and Strong
Acids. When we neutralize a weak base with a strong acid, the product is a
salt containing the conjugate acid of the weak base. This conjugate acid is a weak acid. For example, ammonium
chloride, NH4Cl, is a salt formed by the reaction of the weak base ammonia with the strong acid HCl:
NH3(aq) + HCl(aq) ⟶ NH4Cl(aq)
A solution of this salt contains ammonium ions and chloride ions. The
chloride ion has no effect on the acidity of the solution since HCl is a strong acid. Chloride is a very weak base
and will not accept a proton to a measurable extent. However, the ammonium ion, the conjugate acid of ammonia,
reacts with water and increases the hydronium ion concentration:
NH4+(aq) + H2O(l) → H3O+(aq) + NH3(aq)
The equilibrium equation for this reaction is simply the ionization
constant. Ka, for the acid NH4+:
[H3O+][NH3] /
[NH4+] = Ka
We will not find a value of Ka for the ammonium ion in Appendix H. However, it is not difficult to determine Ka for NH4+ from the value of the ionization constant of water, Kw, and Kb (1), the ionization constant of its conjugate base, NH3,
using the following relationship [2, p. 806]:
Kw = Ka × Kb (1)
Salts of Weak bases and Strong acids. Some salts of weak bases and strong acids undergo cationic hydrolysis
and yield slightly acidic solutions. Ammonium chloride is a typical example of this class of salts. It is the salt of a weak
base. NH4OH, and strong acid, HCl. It ionises in aqueous solution to
form the cation, NH4+.
NH4OH ↔ NH4+ + OH-
conjugate
acid
NH4+ is a Bronsted conjugate acid of the weak base NH4OH.
Therefore, it is a relatively strong acid. It accepts OH– ion from water
(H2O) and forms the unionised NH4OH and H+
ion.
NH4+ + H2O ↔ NH4OH + H+
The accumulation of H+ ions in solution makes it
acidic.
The other examples of this type of salts are ferric chloride,
aluminium chloride, and copper sulphate [24, p. 3].
Salts of Weak acids and Weak bases. The examples of this type of salts are ammonium acetate, ammonium
cyanide and ammonium fluoride. Both the anion and the cation produced by ionisation of the
salt undergo hydrolysis. The resulting solution is neutral, basic or acidic depending on the
relative hydrolysis of the anions and the cations.
Ammonium acetate,CH3COONH4. It is the salt of weak acid, CH3COOH, and weak base,
NH4OH. In aqueous solution it ionises to form the anion
CH3COO– and the cation NH4+. Since the acid and the base are both weak, their conjugate base
(CH3COO–) and conjugate acid ( NH4+) are relatively strong. They accept H+ and OH–
ions respectively from water and undergo considerable hydrolysis.
CH3COO- + H2O ↔ CH3COOH + OH- ...(1)
conjugate base
NH4+ + H2O ↔ NH4OH + H+ ...(2)
conjugate acid
The overall hydrolysis may be represented as
CH3COO- + NH4+ +
H2O ↔ CH3COOH + NH4OH
We have stated above that pH of the resulting solution will depend on
the relative extent of anionic hydrolysis (1) and cationic hydrolysis (2). If
both the ions react to the same extent (as shown for
CH3COONH4), [OH–] = [H+] and the
solution is neutral. If the cation reacts to a greater extent, the solution is
slightly acidic. If the anion is a little more reactive, the solution will be
basic. Thus, a solution of CH3COONH4 is neutral, a
solution of NH4CN is slightly basic and a solution of NH4F
is slightly acidic [24, p. 3].
Equilibrium in a Solution of a Salt of a Weak Acid and a Weak Base.
In a solution of a salt formed by the reaction of a weak acid and a
weak base, to predict the pH, we must know both the Ka of the weak acid and the Kb of the weak base. If Ka > Kb, the solution is acidic, and if Kb > Ka, the solution is basic [2, p. 810].
The Ionization of Hydrated Metal Ions.
If we measure the pH of the solutions of a variety of metal ions we
will find that these ions act as weak acids when in solution. The aluminum ion is an example. When aluminum nitrate
dissolves in water, the aluminum ion reacts with water to give a hydrated aluminum ion, Al(H2O)63+, dissolved in bulk water. What this means is that the aluminum ion has the strongest interactions with the six closest
water molecules (the so-called first solvation shell), even though it does interact with the other water molecules
surrounding this Al(H2O)63+ cluster as well:
Al(NO3)3 (s) + 6H2O (l) ⟶ Al(H2O)63+ (aq) + 3NO3− (aq)
We frequently see the formula of this ion simply as “Al3+
(aq)”, without explicitly noting the six water molecules that are the
closest ones to the aluminum ion and just describing the ion as being solvated
in water (hydrated). This is similar to the simplification of the formula of the
hydronium ion, H3O+ to H+. However, in this
case, the hydrated aluminum ion is a weak acid (figure 8.2) and donates a proton to a water molecule. Thus, the hydration
becomes important and we may use formulas that show the extent of
hydration:
Al(H2O)63+ (aq) + H2O (l) → H3O+ (aq) + Al(H2O)5(OH)2+ (aq) Ka = 1.4 × 10−5
As with other polyprotic acids, the hydrated aluminum ion ionizes in
stages, as shown by:
Al(H2O)63+ (aq) + H2O(l) → H3O+(aq) + Al(H2O)5(OH)2+(aq)
Al(H2O)5(OH)2+ (aq) + H2O(l) → H3O+(aq) + Al(H2O)4(OH)2+(aq)
Al(H2O)4(OH)2+ (aq) + H2O(l) → H3O+(aq) + Al(H2O)3(OH)3(aq)
Note that some of these aluminum species are exhibiting amphiprotic
behavior, since they are acting as acids when they appear on the left side of
the equilibrium expressions and as bases when they appear on the right
side.
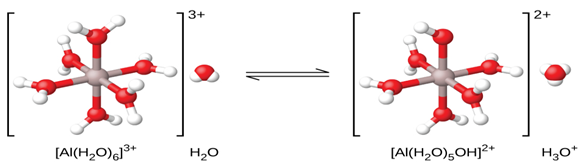
Figure 8.2 When an aluminum ion reacts with water, the hydrated aluminum ion
becomes a weak acid.
However, the ionization of a cation carrying more than one charge is
usually not extensive beyond the first stage. Additional examples of the first
stage in the ionization of hydrated metal ions are:
Fe(H2O)63+(aq) + H2O(l) → H3O+(aq) + Fe(H2O)5(OH)2+(aq) Ka = 2.74
Cu(H2O)62+(aq) + H2O(l) → H3O+(aq) + Cu(H2O)5(OH)+(aq) Ka = ~6.3
Zn(H2O)42+(aq) + H2O(l) → H3O+(aq) + Zn(H2O)3(OH)+(aq) Ka = 9.6
The constants for the different stages of ionization are not known
for many metal ions, so we cannot calculate the extent of their ionization.
However, practically all hydrated metal ions other than those of the alkali
metals ionize to give acidic solutions. Ionization increases as the charge of
the metal ion increases or as the size of the metal ion decreases [2, p.
810].