Solutions
Introduction. Solutions are homogeneous (single-phase) mixtures of two or more
components. They are extremely important in Chemistry because they allow
intimate and varied encounters between molecules of different kinds, a condition
that is essential for rapid chemical reactions to occur. Several more explicit
reasons can be cited for devoting a significant amount of time to the subject of
solutions:
1.
For the reason stated above, most chemical reactions that are carried
out in the laboratory and in industry, and that occur in living organisms, take
place in solution.
2.
Solutions are so common; very few pure substances are found in
nature.
3.
Solutions provide a convenient and accurate means of introducing
known small amounts of a substance to a reaction system. Advantage is taken of
this in the process of titration, for example.
4.
The physical properties of solutions are sensitively influenced by
the balance between the intermolecular forces of like and unlike (solvent and
solute) molecules. The physical properties of solutions thus serve as useful
experimental probes of these intermolecular forces.
That these intermolecular forces can have dramatic effects is made
readily apparent by a simple example. At 0̊ C and 1 atm pressure, one litre of
water will dissolve 1300 litres of ammonia gas; this corresponds to a solubility
of 58 mol L−1. Now if 58 mol of an ideal gas were compressed so that it would fit
into the same 1-liter volume (but without the water), a very high
pressure would be required.
If we actually attempt to compress pure NH3 gas to the
same degree, it would liquid, and the vapor pressure of the liquid would be
about 9 atm. In other words, the escaping tendency of NH3 molecules
from H2O is only about 1/9th of what it is from liquid
NH3. One way of interpreting this is that the strong intermolecular
(dipole-dipole) attractions between NH3 and the solvent
H2O give rise to a force that has the effect of a “negative pressure”
of 9 atm [23, p. 3].
Types of solutions. We usually think of a solution as a liquid; a mixture of a gas,
liquid, or solid solute in a liquid solvent. Actually, solutions can exist as gases and solids as well. Gaseous
mixtures don’t require any special consideration beyond what you learned about
Dalton’s Law earlier in the course. Solid solutions are very common; most
natural minerals and many alloys are solid solutions.
Everyone knows that “oil and water don’t mix”. Actually, this is not
strictly correct, since all substances have at least a slight tendency to
dissolve in each other. This raises two important and related questions: why do
solutions tend to form in the first place, and what factors limit their mutual
solubilities?
You may recall that in the earlier unit on phase equilibria, we
pointed out that aggregations of molecules that are more disordered tend to be the ones that are favored at higher temperature, whereas
those that possess the lowest
potential energy are favored at lower temperatures (table 7.1). This is a
general principle that applies throughout the world of matter; the stable form
at any given temperature will always be that which leads to the best balance
between low potential energy and high molecular disorder.
Table 7.1
Energetics of solution formation
|
Solute |
Solvent |
Energy to disperse solute |
Energy to introduce into solvent |
Increase in randomness |
|
Gas |
Gas |
nil |
nil |
large |
|
liquid or solid |
Gas |
large |
nil |
large |
|
Gas |
Liquid |
nil |
varies |
Negative |
|
Gas |
Solid |
nil |
endothermic |
negative |
|
Liquid |
Liquid |
variable |
variable |
moderate |
|
ionic solid |
polar liquid |
large |
exothermic |
small |
|
molecular solid |
polar liquid |
moderate |
moderate |
moderate |
|
Molecular |
Solid |
nonpolar liquid |
moderate small |
moderate |
|
ionic or covalent solid |
nonpolar liquid |
large |
small |
small |
To see how these considerations are applied to solutions, think about
the individual steps that must be carried out when a solute is dissolved in a
solvent:
1.
The solute must be dispersed; that is, its molecular units must be pulled apart. This
requires energy, and so this step always works against solution formation.
2.
The solute must be introduced into the solvent. Whether this is energetically favorable or unfavorable depends on the
nature of the solute and solvent. If the solute is A and the solvent is B, then
what is important is the strength of the attractive forces between A-A and B-B
molecules, compared to those between A-B pairs; if the latter are greater, then
the potential energy will be lower when the substances are mixed and solution
formation will be favored.
If the sum of these two steps is exothermic, this will favor solution
formation, but there is one other factor to consider: Would the formation of a
solution lead to an increase or a decrease in disorder on the molecular
scale? The answer varies from case to case; mixing of different kinds of
molecules always creates disorder, and substantially increasing the volume they
occupy (as when a solid dissolves in a liquid) can have a very large effect.
Higher temperatures generally favor processes in which disorder is created, but
tend to work against those in which disorder is lost [23, p.
3].
Gaseous solutions. Since the energy associated with the mixing of two gases is
negligible, the tendency to greater disorder dominates at all temperatures, and
gases are miscible in all proportions. When the solute is a solid, “dissolving”
it in a gas is formally equivalent to sublimation. The energy required to remove
the molecules from their neighbors is generally too great to be compensated by
the greater disorder in the vapor phase, so solids tend to have relatively low
vapor pressures. The same is true of liquids at temperatures well below their
boiling points [23, p. 5].
Solutions of gases in liquids.
When a gas dissolves in a liquid, the confinement of the gas in the
much smaller volume of the liquid causes a loss in molecular disorder that is
not usually compensated by the presence of two kinds of molecules in the liquid
phase. Such processes are not favored unless there are strong compensating
factors; gases therefore tend to be only slightly soluble in liquids. Still,
there is always some solubility, for several common gases in water. The greatest
solubilities occur when the gas reacts chemically with the solvent (a
“compensating factor”), as happens, for example, with CO2, HCl, SO2, and especially NH3 in water. In these cases, the fall in potential energy associated
with the reaction helps overcome the unfavorable randomness effect. One important consequence of the
reduction in disorder when a gas dissolves in a liquid is that the solubility of
a gas decreases at higher temperatures; this is in contrast to most other situations,
where a rise in temperature usually leads to increased
solubility.
The temperature dependence of the solubility of oxygen in water is an
important consideration for the well-being of aquatic life; thermal pollution of
natural waters (due to the influx of cooling water from power plants) has been
known to reduce the dissolved oxygen concentration to levels low enough to kill
fish. The advent of summer temperatures in a river can have the same effect if
the oxygen concentration has already been partially depleted by reaction with
organic pollutants [23, p. 5].
Solutions of liquids in liquids. Whereas all gases will mix to form solutions regardless of the
proportions, liquids are much more fussy. Some liquids, such as ethyl alcohol and water, are miscible in
all proportions. Others, like the proverbial “oil and water”, are not; each liquid has only a
limited solubility in the other, and once either of these limits is exceeded,
the mixture separates into two phases.
The reason for this variability is apparent from. Mixing of two
liquids can be exothermic, endothermic, or without thermal effect, depending on
the particular substances. Whatever the case, the energy factors are not usually
very large, but neither is the increase in randomness; the two factors are
frequently sufficiently balanced to produce limited miscibility.
A useful general rule is that liquids are completely miscible when
their intermolecular forces are very similar in nature; “like dissolves like”.
Thus water is miscible with other liquids that can engage in hydrogen bonding,
whereas a hydrocarbon liquid in which London or dispersion forces are the only
significant intermolecular effect will only be completely miscible with similar
kinds of liquids.
Substances such as the alcohols, CH3(CH2)nOH, which are hydrogen-bonding (and thus hydrophilic) at one end and
hydrophobic at the other, tend to be at least partially miscible with both kinds
of solvents. If n is large, the hydrocarbon properties dominate and the alcohol has
only a limited solubility in water. Very small values of n allow the -OH group to dominate, so miscibility in water increases
and becomes unlimited in ethanol (n = 1) and methanol (n = 0), but miscibility with hydrocarbons decreases owing to the energy
required to break alcohol-alcohol hydrogen bonds when the nonpolar liquid is
added.
These considerations have become quite important in the development
of alternative automotive fuels based on mixing these alcohols with gasoline. At
ordinary temperatures the increased randomness of the mixture is great enough
that the unfavorable energy factor is entirely overcome, and the mixture is
completely miscible. At low temperatures, the randomness factor becomes less
predominant, and the fuel mixture may separate into two phases, presenting
severe problems to the fuel filter and carburetor [23, p. 6].
Solutions of molecular solids in liquids. The stronger intermolecular forces in solids require more input of
energy in order to disperse the molecular units into a liquid solution, but there is also a
considerable increase in disorder that can more than compensate if the intermolecular forces are not too
strong, and if the solvent has no strong hydrogen bonds that must be broken in order to introduce
the solute into the liquid.
For example, at 25 ◦C and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml
of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of
iodine. As the molecular weight of the solid increases, the intermolecular
forces holding the solid together also increase, and solubilities tend to fall
off; thus the solid linear hydrocarbons CH3(CH2)nCH3 (n > 20) show diminishing solubilities in hydrocarbon liquids [1, p.
6].
Solutions of ionic solids in water. Since the coulombic forces that bind ions and highly polar molecules
into solids are quite strong, we might expect these solids to be insoluble in
just about any solvent. Ionic solids are insoluble in most non-aqueous solvents,
but the high solubility of some (including NaCl) in water suggests the need for
some further explanation.
The key factor here turns out to be the interaction of the ions with
the solvent. The electrically-charged ions exert a strong coulombic attraction
on the end of the water molecule that has the opposite partial charge. As a
consequence, ions in solution are always hydrated; that is, they are
quite tightly bound to water molecules through ion-dipole interaction. The
number of water molecules contained in the primary hydration shell varies
with the radius and charge of the ion.
The dissolution of an ionic solid in water can be thought of as a
sequence of two steps:
1)
MX(s) → M+(g) + X−(g) ΔH >0 (lattice energy)
2)
M+(g) + X−(g) + H2O → M+(aq) + X−(aq) ΔH <0 (hydration energy)
The first reaction is endothermic, but the second is exothermic;
whether the overall dissolution process is energetically favorable or
unfavorable depends on the actual number of kilojoules associated with the two
processes.
The net energy (the heat of solution) is the sum of two quantities
having large magnitudes and opposite signs; the sign of the result is therefore
rather hard to predict.
The balance between the lattice energy and hydration energy is a
major factor in determining the solubility of an ionic crystal in water, but
there is another factor to consider as well. We generally assume that there is a
rather large increase in disorder when a solid is dispersed into the liquid
phase. However, in the case of ionic solids, each ion ends up surrounded by a
shell of oriented water molecules. The ions themselves have become more
disordered, but the water molecules are now more ordered than before. In some
cases the latter effect predominates, and dissolution of the salt leads to a net
increase in order. Recall that any process in which the net disorder diminishes
becomes less probable as the temperature increases; this explains why the
solubilities of some salts decrease with temperature [23, p.
6].
Solubility and the Solution Process. The amount of substance that will dissolve in a solvent depends on
both the substance and the solvent. We describe the amount that dissolves in terms of
solubility.
Solubility. Saturated Solutions. To understand the concept of solubility, consider the process of
dissolving sodium chloride in water. Sodium chloride is an ionic substance, and it
dissolves in water as Na+ and Cl- ions. If you could view the dissolving of sodium chloride at the
level of ions, you would see a dynamic process. Suppose you stir 40.0 g of
sodium chloride crystals into 100 ml of water at 20ºC. Sodium ions and chloride
ions leave the surface of the crystals and enter the solution. The ions move
about at random in the solution and may by chance collide with a crystal and
stick, thus returning to the crystalline state. As the sodium chloride continues
to dissolve, more ions enter the solution, and the rate at which they return to
the crystalline state increases (the more ions in solution, the more likely ions
are to collide with the crystals and stick). Eventually, a dynamic equilibrium is reached in which the rate at which ions leave the crystals equals
the rate at which ions return to the crystals. You write the dynamic equilibrium
this way:
NaCl(s) → N+(aq) +
Cl-(aq)
At equilibrium, no more sodium chloride appears to dissolve; 36.0 g
has gone into solution, leaving 4.0 g of crystals at the bottom of the vessel.
You have a saturated solution—that is, a solution that is in equilibrium with
respect to a given dissolved substance. The solution is saturated
with respect to NaCl, and no more NaCl can dissolve. The solubility of sodium chloride in water
(the amount that dissolves
in a given quantity of water at a
given temperature to give a saturated solution) is 36.0 g/100 mL at 20ºC.
Note that if you had mixed 30.0 g of sodium chloride with 100 ml of water, all
of the crystals would have dissolved. You would have an unsaturated solution, a solution not in equilibrium with respect
to a given dissolved substance
and in which more of the substance can dissolve.
Sometimes it is possible to obtain a supersaturated solution, a solution that contains more dissolved
substance than a saturated solution does. For example, the solubility of sodium thiosulfate,
Na2S2O3, in water at 100ºC is 231 g/100 ml. But
at room temperature, the
solubility is much less—about 50 g/100 ml. Suppose you prepare a solution saturated with sodium
thiosulfate at 100ºC. You might expect that as the water solution was cooled,
sodium thiosulfate would crystallize out. In fact, if the solution is slowly
cooled to room temperature, this does not occur. Instead the result is a
solution in which 231 g of sodium thiosulfate is dissolved in 100 ml of cold
water, compared with the 50 g you would normally expect to find dissolved.
Supersaturated solutions are not in equilibrium with the solid
substance. If a small crystal of sodium thiosulfate is added to a supersaturated
solution, the excess immediately crystallizes out. Crystallization from a
supersaturated solution is usually quite fast and
dramatic.
Factors in Explaining Solubility. The solubilities of substances in one another vary widely. You might
find a substance miscible in one solvent but nearly insoluble in another. As a general
rule, “like dissolves like.” That is, similar substances dissolve one
another. Oil is miscible in gasoline. Both are mixtures of hydrocarbon substances (compounds
of hydrogen and carbon only). On the other hand, oil does not mix with water.
Water is a polar substance, whereas hydrocarbons are not. Why do similar
substances dissolve in one another to greater extents than do dissimilar
substances? What factors are involved in solubility?
The solubility of
one substance in another can be explained in terms of two factors. One is the
natural tendency of substances to mix. This is sometimes also referred to as the
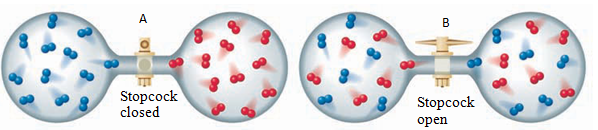
natural tendency toward disorder. Figure 7.1A shows a vessel divided into
two parts, with oxygen gas on the left and nitrogen gas on the right. If you
remove the partition, the molecules of the two gases begin to mix. Ultimately,
the molecules become thoroughly mixed through their random motions (figure
7.1B). You might expect a similar mixing of molecules or ions in other
types of solutions.
If the process of
dissolving one substance in another involved nothing more than simple mixing,
you would expect substances to be completely soluble in one another; that is,
you would expect substances to be miscible. You know that this is only sometimes
the case. Usually, substances have limited solubility in one another. A factor
that can limit solubility is the relative forces of attraction between species
(molecules or ions). Suppose there are strong attractions between solute species
and strong attractions between solvent species, but weak attractions between
solute and solvent species. In that case, the strongest attractions are
maintained so long as the solute and solvent species do not mix. The lowest
energy of the solute–solvent system is obtained then also.
The solubility of a solute in a solvent (that is, the extent of the
mixing of the solute and solvent species) depends on a balance between the
natural tendency for the solute and solvent species to mix and the tendency for
a system to have the lowest energy possible.
Molecular Solutions. The simplest example of a molecular solution is one gas dissolved in
another gas. Air, essentially a solution of oxygen and nitrogen, is an example. The
intermolecular forces in gases are weak. The only solubility factor of importance is the
tendency for molecules to mix. Gases are therefore miscible.
Substances may be miscible even when the intermolecular forces are
not negligible. Consider the solution of the two similar liquid hydrocarbons heptane,
C7H16, and octane, C8H18, which are components of gasoline. The
intermolecular attractions are due to London forces, and those between heptane and octane molecules are
nearly equal to those between octane and octane molecules and heptane and heptane
molecules. The different intermolecular attractions are about the same strength, so
there are no favored attractions. Octane and heptane molecules tend to move
freely through one another.
As a counterexample, consider the mixing of octane with water. There
are strong hydrogen bonding forces between water molecules. For octane to mix
with water, hydrogen bonds must be broken and replaced by much weaker London
forces between water and octane. In this case, the maximum forces of attraction
among molecules (and therefore the lower energy) result if the octane and water
remain unmixed. Therefore, octane and water are nearly immiscible.
The statement “like dissolves like” succinctly expresses these
observations. That is, substances with similar intermolecular attractions are
usually soluble in one another. The two similar hydrocarbons heptane and octane
are completely miscible, whereas octane and water (with dissimilar
intermolecular attractions) are immiscible. The solubility in water decreases
from miscible to slightly soluble. Water and alcohols are alike in having —OH
groups through which strong hydrogen bonding attractions arise.
The attraction between a methanol molecule, CH3OH, and a
water molecule is nearly as strong as that between two methanol molecules or
between two water molecules. Methanol and water molecules tend to mix freely.
Methanol and water are miscible, as are ethanol and water and 1-propanol and
water. However, as the hydrocarbon end, R—, of the alcohol becomes the more
prominent portion of the molecule, the alcohol becomes less like water. Now the
forces of attraction between alcohol and water molecules are weaker than those
between two alcohol molecules or between two water molecules. Therefore, the
solubilities of alcohols decrease with increasing length of R [15, p.
524].
Ionic Solutions. Ionic substances differ markedly in their solubilities in water. For
example, sodium chloride, NaCl, has a solubility of 36 g per 100 ml of water at room
temperature, whereas calcium phosphate,
Ca3(PO4)2, has a solubility of only 0.002 g per
100 ml of water. In most cases, these differences in solubility can be
explained in terms of the different energies of attraction between ions in the crystal
and between ions and water.
The energy of attraction between an ion and a water molecule is due
to an ion–dipole force. Water molecules are polar, so they tend to orient with respect to
nearby ions. In the case of a positive ion (Li+, for example), water molecules orient with their oxygen atoms (the
negative ends of the molecular dipoles) toward the ion. In the case of a negative ion (for instance, F-),
water molecules orient with their hydrogen atoms (the positive ends of the
molecular dipoles) toward the ion. The attraction of ions for water molecules
is called hydration. Hydration of ions favors the dissolving
of an ionic solid in water. Ions on the surface become hydrated and then
move into the body of the solution as hydrated ions. If the
hydration of ions were the only factor in the solution process, you
would expect all ionic solids to be soluble in water. The ions in
a crystal, however, are very strongly attracted to one another.
Therefore, the solubility of an ionic solid depends not only on
the energy of hydration of ions (energy associated with the attraction
between ions and water molecules) but also on lattice energy,
the energy holding ions together in the crystal lattice. Lattice
energy works against the solution process, so an ionic solid with
relatively large lattice energy is usually insoluble. < Lattice
energies depend on the charges on the ions (as well as on the distance
between the centers of neighboring positive and negative ions). The greater
the magnitude of ion charge, the greater is the lattice energy. < For
this reason, you might expect substances with singly charged ions to be
comparatively soluble and those with multiply charged ions to be less soluble.
This is borne out by the fact that compounds of the alkali metal ions (such as
Na+ and K+) and ammonium ions (NH4+)
are generally soluble, whereas those of phosphate ions
(PO43-), for example, are generally
insoluble.
Lattice energy is also inversely proportional to the distance between
neighboring ions, and this distance depends on the sum of the radii of the ions.
For example, the lattice energy of magnesium hydroxide, Mg(OH)2, is
inversely proportional to the sum of the radii of Mg2+ and
OH-. In the series of alkaline earth hydroxides— Mg(OH)2,
Ca(OH)2, Sr(OH)2, Ba(OH)2—the lattice energy
decreases as the radius of the alkaline earth ion increases (from
Mg2+ to Ba2+). If the lattice energy alone determines the
trend in solubilities, you should expect the solubility to increase from
magnesium hydroxide to barium hydroxide. In fact, this is what you find.
Magnesium hydroxide is insoluble in water, and barium hydroxide is soluble. But
this is not the whole story. The energy of hydration also depends on ionic
radius. A small ion has a concentrated electric charge and a strong electric
field that attracts water molecules. Therefore, the energy of hydration is
greatest for a small ion such as Mg2+ and least for a large ion such
as Ba2+. If the energy of hydration of ions alone determined the trend in solubilities, you would expect the
solubilities to decrease from magnesium hydroxide to barium hydroxide, rather
than to increase.
The explanation for the observed solubility trend in the alkaline
earth hydroxides is that the lattice energy decreases more rapidly in the series
Mg(OH)2, Ca(OH)2, Sr(OH)2, and
Ba(OH)2 than does the energy of hydration in the series of ions
Mg2+, Ca2+, Sr2+, and Ba2+. For this
reason, the lattice-energy factor dominates this solubility
trend.
You see the opposite solubility trend when the energy of hydration
decreases more rapidly so that it dominates the trend. Consider the alkaline
earth sulfates. Here the lattice energy depends on the sum of the radius of the
cation and the radius of the sulfate ion. Because the sulfate ion,
SO42-, is much larger than the hydroxide ion,
OH-, the percent change in lattice energy in going through the series
of sulfates from MgSO4 to BaSO4 is smaller than in the
hydroxides. The lattice energy changes less, and the energy of hydration of the
cation decreases by a greater amount. Now the energy of hydration dominates the
solubility trend, and the solubility decreases from magnesium sulfate to barium
sulfate. Magnesium sulfate is soluble in water, and barium sulfate is insoluble
[15, p. 525].
Effects of Temperature and Pressure on Solubility. In general, the solubility of a substance depends on temperature. For
example, the solubility of ammonium nitrate in 100 ml of water is 118 g at 0ºC
and 811 g at 100ºC. Pressure may also have an effect on solubility, as you will
see.
Temperature Change. Most gases become less soluble in water at higher temperatures. The
first bubbles that appear when tap water is heated are bubbles of air released as the
increasing temperature reduces the solubility of air in water. In contrast, most ionic
solids become more soluble in water with rising temperature.
The variations of the solubilities of the salts KNO3,
CuSO4, NaCl, and Ce2(SeO4)3. Three
of the salts show the usual behavior; their solubilities increase with rising
temperature. For example, potassium nitrate, KNO3, changes solubility
dramatically from 14 g/100 g H2O at 0ºC to 245 g/100 g H2O
at 100ºC. Copper (II) sulfate, CuSO4, shows a moderate increase in
solubility over this temperature interval. Sodium chloride, NaCl, increases only
slightly in solubility with temperature.
A number of ionic compounds decrease in solubility with increasing
temperature. Calcium sulfate, CaSO4, and calcium hydroxide,
Ca(OH)2, are common examples. They are slightly soluble compounds
that become even less soluble at higher temperatures. Cerium selenate,
Ce2(SeO4)3, is very soluble at 0ºC but much
less soluble at 100ºC.
Heat can be released or absorbed when ionic substances are dissolved
in water. In some cases, this heat of solution is quite noticeable. When
sodium hydroxide is dissolved in water, the solution becomes hot (the solution
process is exothermic). On the other hand, when ammonium nitrate is dissolved in
water, the solution becomes very cold (the solution process is endothermic).
This cooling effect from the dissolving of ammonium nitrate in water is
exploited in instant cold packs used in hospitals and elsewhere. An instant cold
pack consists of a bag of NH4NO3 crystals inside a bag of
water. When the inner bag is broken, NH4NO3 dissolves in
the water. Heat is absorbed, so the bag feels cold. Hot packs, by contrast,
containing either CaCl2 or MgSO4, dissolve in water with
the evolution of heat [15, p. 528].
Pressure Change. In general, pressure change has little effect on the solubility of a
liquid or solid in water, but the solubility of a gas is very much affected by pressure.
The qualitative effect of a change in pressure on the solubility of a gas can be
predicted from Le Chatelier’s principle. Le Chatelier’s principle states that when a system in equilibrium is disturbed by a change of temperature, pressure, or concentration
variable, the system shifts in equilibrium composition in a way that tends to counteract
this change of variable. Let us see how Le Chatelier’s principle can predict the effect of a
change in pressure on gas solubility.
Imagine a cylindrical vessel that is fitted with a movable piston and
contains carbondioxide gas over its saturated water solution. The equilibrium
is
CO2 (g) ↔ CO2 (aq)
Suppose you increase the partial pressure of CO2 gas by
pushing the piston down. This change of partial pressure, according to Le
Chatelier’s principle, shifts the equilibrium composition in a way that tends to
counteract the pressure increase. From the preceding equation, you see that the
partial pressure of CO2 gas decreases if more CO2
dissolves.
CO2 (g) →
CO2 (aq)
The system comes to a new equilibrium, in which more CO2
has dissolved. So you can predict that carbon dioxide is more soluble at higher
pressures. Conversely, when the partial pressure of carbon dioxide gas is
reduced, its solubility is decreased. A bottle of carbonated beverage fizzes
when the cap is removed: as the partial pressure of carbon dioxide is reduced,
gas comes out of solution. The argument given here for carbon dioxide holds for
any gas: All gases become more soluble in a liquid at a given temperature when
the partial pressure of the gas over the solution is increased [15, p.
529].
Henry’s Law: Relating Pressure to the Solubility of a Gas in a
Liquid. The effect of pressure on the solubility of a gas in a liquid can be
predicted quantitatively. According to Henry’s law, the solubility of a gas is directly proportional to the partial pressure of the gas above the solution. Expressed mathematically, the law is (1)
S = kHP (1)
where S is the solubility of the gas (expressed as mass of solute per unit
volume of solvent), kH is Henry’s law constant for the gas for a particular liquid at a
given temperature, and P is the partial pressure of the gas. The next example shows how this
formula is used. In the chapter opening, we mentioned that the addition of
ethylene glycol, CH2OHCH2OH, to water lowers the freezing
point of water below 0ºC. For example, if 0.010 mol of ethylene glycol is added
to 1 kg of water, the freezing point is lowered to -0.019ºC. The magnitude of
freezing-point lowering is directly proportional to the number of ethylene
glycol molecules added to a quantity of water. Thus, if you add 0.020 (= 0.010 ×
2) mol of ethylene glycol to 1 kg of water, the freezing point is lowered to
-0.038ºC (= -0.019ºC × 2). The same lowering is observed with the addition of
other nonelectrolyte substances. For example, an aqueous solution of 0.020 mol
of urea, (NH2)2CO, in 1 kg of water also freezes at
-0.038ºC.
Freezing-point lowering is a colligative property. Colligative
properties of solutions are properties that depend on the concentration
of solute molecules or ions in solution but not on the chemical identity of the
solute (whether it is ethylene glycol or urea, for instance). In the next
sections, we will discuss several colligative properties, which are expressed quantitatively
in terms of various concentration units. We will first look into ways of
expressing the concentration of a solution.
Ways of Expressing Concentration.
Molarity. The concentration of a solute is the amount of solute dissolved in a given quantity
of solvent or solution. The quantity of solvent or solution can be
expressed in terms of volume or in terms of mass or molar amount. Thus, there are several
ways of expressing the concentration of a solution.
Recall that the molarity of a solution is the moles of solute in a liter of solution (2).
Molarity = moles of solute / liters of solution
(2)
For example, 0.20 mol of ethylene glycol dissolved in enough water to
give 2.0 L of solution has a molarity of
0,20 mol ethylene glycol / 2,0 L solution = 0,10 M ethylene
glycol
The solution is 0.10 molar (denoted M). This unit is especially useful when you wish to dispense a given
amount of solute, because the unit directly relates the amount of solute to the
volume of solution.
Some other concentration units are defined in terms of the mass or
molar amount of solvent or solution. The most important of these are mass
percentage of solute, molality, and mole fraction [15, p.
531].
Mass Percentage of Solute. Solution concentration is sometimes expressed in terms of the
mass percentage of solute—that is, the percentage by mass of solute contained in a solution
(3).
Mass percentage of solute = mass of solute / mass of solution × 100%
(3)
For example, an aqueous solution that is 3.5% sodium chloride by mass
contains 3.5 g of NaCl in 100.0 g of solution. It could be prepared by
dissolving 3.5 g of NaCl in 96.5 g of water (100.0 - 3.5 = 96.5) [15, p. 532].
Molality. The molality of a solution is the moles of solute per kilogram of solvent
(4).
Molality = moles of solute / kilograms of solvent (4)
For example, 0.20 mol of ethylene glycol dissolved in 2.0 ×
103 g (= 2.0 kg) of water has a molality of
0,20 mol ethylene glycol / 2,0 kg solvent = 0,10 m ethylene
glycol
That is, the solution is 0.10 molal (denoted m). The units of molality and
molarity are sometimes confused. Note that molality is defined in terms of mass of solvent, and molarity is
defined in terms of volume of solution
[15, p. 532].
Mole Fraction. The mole fraction of a component substance A (XA) in a solution is defined as the moles of component substance divided by the total moles of
solution (that is, moles of solute plus solvent) (5).
XA = moles of substance A / total moles of solution
(5)
For example, if a solution is made up of 1 mol of ethylene glycol and
9 mol of water, the total moles of solution are 1 mol + 9 mol = 10 mol. The mole fraction of ethylene glycol is 1/10 = 0.1, and the mole fraction of water is 9/10 = 0.9. Multiplying mole fractions by 100 gives mole percent. Hence, this solution is 10 mole percent ethylene glycol and 90 mole
percent water. You can also say that 10% of the molecules in the solution are
ethylene glycol and 90% are water. The sum of the mole fractions of all the
components of a solution equals 1 [15, p. 534].