Energy and the direction of chemical process
Energy. Energy can be defined as the capacity to supply heat or do work. One type of
work (w) is the process of causing
matter to move against an opposing force. For example, we do work when we
inflate a bicycle tire—we move matter
(the air in the pump) against the opposing force of the air already in
the tire
Like matter, energy comes in different types. One scheme classifies
energy into two types: potential energy, the energy an object has because of its relative position,
composition, or condition, and kinetic energy, the energy that an object possesses because of its motion. Water at
the top of a waterfall or dam has potential energy because of its position; when
it flows downward through generators, it has kinetic energy that can be used to
do work and produce electricity in a hydroelectric plant (figure 6.1). A battery has potential
energy because the chemicals within it can produce electricity that can do
work.

Figure 6.1 (a) Water that is higher in elevation, for example, at the top of
Victoria Falls, has a higher potential energy than water at a lower elevation.
As the water falls, some of its potential energy is converted into kinetic
energy. (b) If the water flows through generators at the bottom of a dam, such
as the Hoover Dam shown here, its kinetic energy is converted into electrical
energy.
Energy can be converted from one form into another, but all of the energy
present before a change occurs always exists in some form after the change is
completed. This observation is expressed in the law of conservation of energy:
during a chemical or physical change, energy can be neither created nor
destroyed, although it can be changed in form. (This is also one version of the
first law of thermodynamics, as you will learn later.)
When one substance is converted into another, there is always an
associated conversion of one form of energy into another. Heat is usually
released or absorbed, but sometimes the conversion involves light, electrical
energy, or some other form of energy. For example, chemical energy (a type of
potential energy) is stored in the molecules that compose gasoline. When
gasoline is combusted within the cylinders of a car’s engine, the rapidly
expanding gaseous products of this chemical reaction generate mechanical energy
(a type of kinetic energy) when they move the cylinders’
pistons.
According to the law of conservation of matter (seen in an earlier
chapter), there is no detectable change in the total amount of matter during a
chemical change. When chemical reactions occur, the energy changes are
relatively modest and the mass changes are too small to measure, so the laws of
conservation of matter and energy hold well. However, in nuclear reactions, the
energy changes are much larger (by factors of a million or so), the mass changes
are measurable, and matter-energy conversions are significant. This will be
examined in more detail in a later chapter on nuclear chemistry. To encompass
both chemical and nuclear changes, we combine these laws into one statement: The
total quantity of matter and energy in the universe is fixed [2, p.
241].
Thermal Energy, Temperature and Heat. Thermal energy is kinetic energy associated with the random motion of atoms and
molecules. Temperature is a quantitative
measure of “hot” or “cold.” When the atoms and molecules in an object are moving
or vibrating quickly, they have
a higher average kinetic energy (KE), and we say that the object is “hot.” When
the atoms and molecules are
moving slowly, they have lower KE, and we say that the object is “cold”
(figure 6.2). Assuming that no
chemical reaction or phase
change (such as melting or vaporizing) occurs, increasing the amount of thermal
energy in a sample of matter
will cause its temperature to increase. And, assuming that no chemical reaction
or phase change (such as condensation or freezing) occurs, decreasing the amount
of thermal energy in a sample of matter will cause its temperature to
decrease.
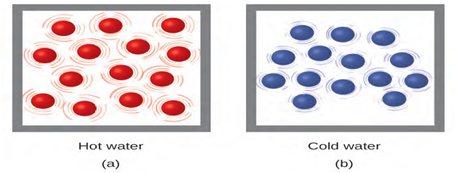
Figure 6.2 (a) The molecules in a sample of hot water move more rapidly than (b)
those in a sample of cold water.
Most substances expand as their temperature increases and contract as
their temperature decreases. This property can be used to measure temperature
changes, as shown in Figure 6.3. The operation of many thermometers depends on the expansion and
contraction of substances in response to temperature
changes.
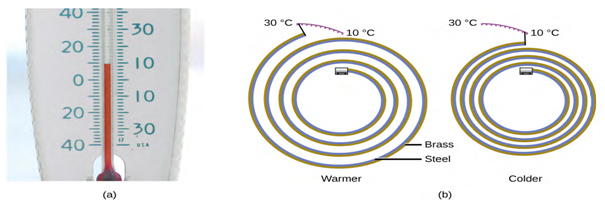
Figure 6.3 (a) In an alcohol or mercury thermometer, the liquid (dyed red for
visibility) expands when heated and contracts when cooled, much more so than the
glass tube that contains the liquid. (b) In a bimetallic thermometer, two
different metals (such as brass and steel) form a two-layered strip. When heated
or cooled, one of the metals (brass) expands or contracts more than the other
metal (steel), causing the strip to coil or uncoil. Both types of thermometers
have a calibrated scale that indicates the temperature.
Heat (q) is the transfer of thermal energy between two bodies at different
temperatures. Heat flow (a redundant term, but one commonly used) increases the
thermal energy of one body and decreases the thermal energy of the other.
Suppose we initially have a high temperature (and high thermal energy) substance
(H) and a low temperature (and low thermal energy) substance (L). The atoms and
molecules in H have a higher average KE than those in L. If we place substance H
in contact with substance L, the thermal energy will flow spontaneously from
substance H to substance L. The temperature of substance H will decrease, as
will the average KE of its molecules; the temperature of substance L will
increase, along with the average KE of its molecules. Heat flow will continue
until the two substances are at the same temperature (figure 6.4).
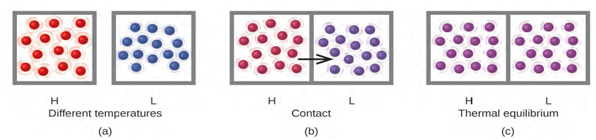
Figure 6.4 (a) Substances H and L are initially at different temperatures, and
their atoms have different average kinetic energies. (b) When they are put into
contact with each other, collisions between the molecules result in the transfer
of kinetic (thermal) energy from the hotter to the cooler matter. (c) The two
objects reach “thermal equilibrium” when both substances are at the same
temperature, and their molecules have the same average kinetic
energy.
Matter undergoing chemical reactions and physical changes can release
or absorb heat. A change that releases heat is called an exothermic process. For example, the combustion reaction that occurs when using an
oxyacetylene torch is an exothermic process—this process also releases energy in
the form of light as evidenced by the torch’s flame. A reaction or change that
absorbs heat is an endothermic process. A cold pack used to treat muscle strains provides an example of an
endothermic process. When the substances in the cold pack (water and a salt like
ammonium nitrate) are brought together, the resulting process absorbs heat,
leading to the sensation of cold.
Exothermic reactions:
·
Heat is transferred from the system to the surroundings.
·
Δ𝐻 is negative.
·
The enthalpy of the products is lower than the enthalpy of the
reactants.
·
The products are energetically more stable than the
reactants.
·
This can be represented in an energy diagram (figure
6.5):
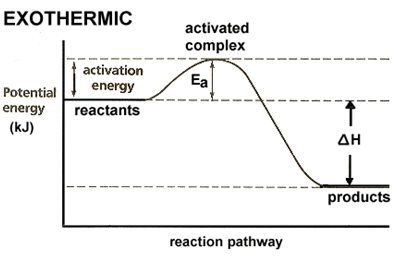
Figure 6.5 Exothermic
Endothermic reactions:
·
Heat is absorbed from
the surroundings.
·
Δ𝐻 is positive.
·
The enthalpy of the products is greater than the enthalpy of the
reactants.
·
The products are energetically less stable that the
reactants.
·
This can also be represented in a similar energy diagram (figure
6.6): [21, p. 1]
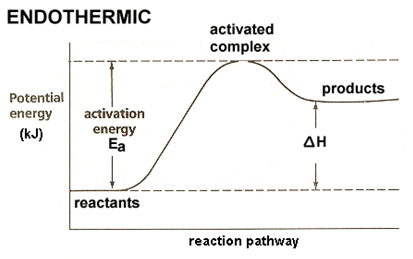
Figure 6.6 Endothermic
Historically, energy was measured in units of calories (cal). A calorie is the amount of energy required to raise one gram of
water by 1 degree C (1 kelvin). However, this quantity depends on the
atmospheric pressure and the starting temperature of the water. The ease of
measurement of energy changes in calories has meant that the calorie is still
frequently used. The Calorie (with a capital C), or large calorie, commonly used
in quantifying food energy content, is a kilocalorie. The SI unit of heat, work,
and energy is the joule. A joule (J) is defined as the amount of energy used when a force of 1 newton
moves an object 1 meter. It is named in honor of the English physicist James
Prescott Joule. One joule is equivalent to 1 kg m2/s2,
which is also called 1 newton–meter. A kilojoule (kJ) is 1000 joules. To
standardize its definition, 1 calorie has been set to equal 4.184
joules.
We now introduce two concepts useful in describing heat flow and
temperature change. The heat capacity (C) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change
(ΔT) of 1 degree Celsius (or equivalently, 1 kelvin)
(1):
C = q/∆T (1)
Heat capacity is determined by both the type and amount of substance
that absorbs or releases heat. It is therefore an extensive property—its value
is proportional to the amount of the substance.
For example, consider the heat capacities of two cast iron frying
pans. The heat capacity of the large pan is five times greater than that of the
small pan because, although both are made of the same material, the mass of the
large pan is five times greater than the mass of the small pan. More mass means
more atoms are present in the larger pan, so it takes more energy to make all of
those atoms vibrate faster. The heat capacity of the small cast iron frying pan
is found by observing that it takes 18,150 J of energy to raise the temperature
of the pan by 50.0 °C:
Csmall pan = 18,140 J/ 50,0 oC = 363 J/oC
The larger cast iron frying pan, while made of the same substance,
requires 90,700 J of energy to raise its temperature by 50.0 °C. The larger pan
has a (proportionally) larger heat capacity because the larger amount of
material requires a (proportionally) larger amount of energy to yield the same
temperature change:
Csmall pan = 90,700 J/ 50,0 oC = 1814 J/oC
The specific heat capacity (c) of a substance, commonly called its “specific heat,” is the quantity
of heat required to raise the temperature of 1 gram of a substance by 1 degree
Celsius (or 1 kelvin) (2):
C = q/m∆T (2)
Specific heat capacity depends only on the kind of substance
absorbing or releasing heat. It is an intensive property—the type, but not the
amount, of the substance is all that matters. For example, the small cast iron
frying pan has a mass of 808 g. The specific heat of iron (the material used to
make the pan) is therefore:
Ciron = 18,140 J/ (808g)(50,0 oC) = 0,449 J/oC
The large frying pan has a mass of 4040 g. Using the data for this
pan, we can also calculate the specific heat of iron:
Ciron = 90,700 J/ (4040g)(50,0 oC) = 0,449
J/oC
Although the large pan is more massive than the small pan, since both
are made of the same material, they both yield the same value for specific heat
(for the material of construction, iron). Note that specific heat is measured in
units of energy per temperature per mass and is an intensive property, being
derived from a ratio of two extensive properties (heat and mass). The molar heat
capacity, also an intensive property, is the heat capacity per mole of a
particular substance and has units of J/mol °C.
Liquid water has a relatively high specific heat (about 4.2 J/g °C);
most metals have much lower specific heats (usually less than 1 J/g °C). The
specific heat of a substance varies somewhat with temperature. However, this
variation is usually small enough that we will treat specific heat as constant
over the range of temperatures that will be considered in this chapter. Specific
heats of some common substances are listed in Table 6.1.
Table 6.1
Specific Heats of Common Substances at 25 °C and 1
bar
|
Substance |
Symbol
(state) |
Specific Heat (J/g °C) |
|
helium |
He(g) |
5.193 |
|
water |
H2O(l) |
4.184 |
|
ethanol |
C2H5OH(l) |
2.376 |
|
ice |
H2O(s) |
2.093
(at −10 °C) |
|
water
vapor |
H2O(g) |
1.864 |
|
nitrogen |
N2(g) |
1.040 |
|
air |
|
1.007 |
|
oxygen |
O2(g) |
0.918 |
|
aluminum |
Al(s) |
0.897 |
|
carbon
dioxide |
CO2(g) |
0.853 |
|
Argon |
Ar(g) |
0.522 |
|
iron |
Fe(s) |
0.449 |
|
copper |
Cu(s) |
0.385 |
|
lead |
Pb(s) |
0.130 |
|
Gold |
Au(s) |
0.129 |
|
silicon |
Si(s) |
0.712 |
If we know the mass of a substance and its specific heat, we can
determine the amount of heat, q, entering or leaving the substance by measuring the temperature
change before and after the heat is gained or lost (3):
q = (specific hea ) × (mass of substance) × (temperature
change)
q = c × m × ΔT = c × m × (Tfina − Tinitial) (3)
In this equation, c is the specific heat of the substance, m is its mass, and ΔT (which is read “delta T”) is the temperature change, Tfinal − Tinitial. If a substance gains thermal energy, its temperature increases, its
final temperature is higher than its initial temperature, Tfinal − Tinitial has a positive value, and the value of q is positive. If a substance loses thermal energy, its temperature
decreases, the final temperature is lower than the initial temperature,
Tfinal − Tinitial has a negative value, and the value of q is negative [2, p. 242].
Enthalpy. Thermochemistry is a branch of chemical thermodynamics, the science that deals with the relationships between heat, work,
and other forms of energy in the context of chemical and physical processes. As
we concentrate on thermochemistry in this chapter, we need to consider some
widely used concepts of thermodynamics.
Substances act as reservoirs of energy, meaning that energy can be
added to them or removed from them. Energy is stored in a substance when the
kinetic energy of its atoms or molecules is raised. The greater kinetic energy
may be in the form of increased translations (travel or straight-line motions),
vibrations, or rotations of the atoms or molecules. When thermal energy is lost,
the intensities of these motions decrease and the kinetic energy falls. The
total of all possible kinds of energy present in a substance is called the
internal energy (U), sometimes symbolized as E.
As a system undergoes a change, its internal energy can change, and
energy can be transferred from the system to the surroundings, or from the
surroundings to the system. Energy is transferred into a system when it absorbs
heat (q) from the surroundings or when the surroundings do work
(w) on the system. For example, energy is transferred into
room-temperature metal wire if it is immersed in hot water (the wire absorbs
heat from the water), or if you rapidly bend the wire back and forth (the wire
becomes warmer because of the work done on it). Both processes increase the
internal energy of the wire, which is reflected in an increase in the wire’s
temperature. Conversely, energy is transferred out of a system when heat is lost
from the system, or when the system does work on the
surroundings.
The relationship between internal energy, heat, and work can be
represented by the equation (4):
ΔU = q + w (4)
as shown in figure 6.7. This is one version of the first law of thermodynamics, and it shows that the internal energy of a system changes through
heat flow into or out of the system (positive q is heat flow in; negative q is heat flow out) or work done on or by the system. The work,
w, is positive if it is done on the system and negative if it is done
by the system.
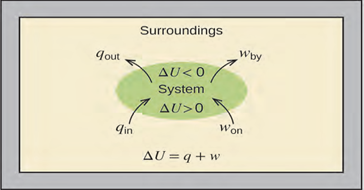
Figure 6.7 The internal energy, U, of a system can be changed by heat flow and work. If heat flows
into the system, qin, or work is done on the system, won, its internal energy increases, ΔU < 0. If heat flows out of the system, qout, or work is done by the system, wby, its internal energy decreases, ΔU > 0.
A type of work called expansion work (or pressure-volume work) occurs when a system pushes back the
surroundings against a restraining pressure, or when the surroundings compress
the system. An example of this occurs during the operation of an internal
combustion engine. The reaction of gasoline and oxygen is exothermic. Some of
this energy is given off as heat, and some does work pushing the piston in the
cylinder. The substances involved in the reaction are the system, and the engine
and the rest of the universe are the surroundings. The system loses energy by
both heating and doing work on the surroundings, and its internal energy
decreases. (The engine is able to keep the car moving because this process is
repeated many times per second while the engine is running.) We will consider
how to determine the amount of work involved in a chemical or physical change in
the chapter on thermodynamics.
Chemists ordinarily use a property known as enthalpy (H) to describe the thermodynamics of chemical and physical processes.
Enthalpy is defined as the sum of a system’s internal energy (U) and the mathematical product of its pressure (P) and volume (V):
H = U + PV
Since it is derived from three state functions (U, P, and V), enthalpy is also a state function. Enthalpy values for specific
substances cannot be measured directly; only enthalpy changes for chemical or physical processes can be determined. For processes
that take place at constant pressure (a common condition for many chemical and
physical changes), the enthalpy change (ΔH) is (5):
ΔH = ΔU + PΔV (5)
The mathematical product PΔV represents work (w), namely, expansion or pressure-volume work as noted. By their
definitions, the arithmetic signs of ΔV and w will always be opposite:
PΔV = −w
Substituting this equation and the definition of internal energy into
the enthalpy-change equation yields:
ΔH = ΔU + PΔV = qp + w – w = qp
where qp is the heat of reaction under conditions of constant pressure. And
so, if a chemical or physical process is carried out at constant pressure with
the only work done caused by expansion or contraction, then the heat flow
(qp) and enthalpy change (ΔH) for the process are equal. The heat given off when you operate a
Bunsen burner is equal to the enthalpy change of the methane combustion reaction
that takes place, since it occurs at the essentially constant pressure of the
atmosphere. On the other hand, the heat produced by a reaction measured in a
bomb calorimeter is not equal to ΔH because the closed, constant-volume metal container prevents
expansion work from occurring. Chemists usually perform experiments under normal
atmospheric conditions, at constant external pressure with q = ΔH, which makes enthalpy the most convenient choice for determining
heat.
The following conventions apply when we use ΔH:
1.
Chemists use a thermochemical equation to represent the changes in
both matter and energy. In a thermochemical equation, the enthalpy change of a
reaction is shown as a ΔH value following the equation for the reaction. This ΔH value indicates the amount of heat associated with the reaction
involving the number of moles of reactants and products as shown in the chemical equation. For example, consider this equation:
H2(g) + 1/2O2(g) ⟶ H2O(l) ΔH = −286 kJ
This equation indicates that when 1 mole of hydrogen gas and
12 mole of oxygen gas at some temperature and pressure change to 1 mole of liquid water at the same temperature and
pressure, 286 kJ of heat are released to the surroundings. If the coefficients of the chemical equation are
multiplied by some factor, the enthalpy change must be multiplied by that same factor (ΔH is an extensive property):
(two-fold increase in amounts)
2H2(g) + O2(g) ⟶ 2H2O(l) ΔH = 2 × (−286 kJ) = −572 kJ
(two-fold decrease in amounts)
1/2H2(g) + 1/4O2(g) ⟶ 12H2O(l) ΔH = 12; × (−286 kJ) = −143 kJ
2.
The enthalpy change of a reaction depends on the physical state of
the reactants and products of the reaction (whether we have gases, liquids,
solids, or aqueous solutions), so these must be shown. For example, when
1mole of hydrogen gas and 12 mole of oxygen gas change to 1 mole of liquid water at the same
temperature and pressure, 286 kJ of heat are released. If gaseous water forms,
only 242 kJ of heat are released.
H2(g) + 1/2O2(g) ⟶ H2O(g) ΔH = −242 kJ
3.
A negative value of an enthalpy change, ΔH, indicates an exothermic reaction; a positive value of ΔH indicates an endothermic reaction. If the direction of a chemical
equation is reversed, the arithmetic sign of its ΔH is changed (a process that is endothermic in one direction is
exothermic in the opposite direction).
Enthalpy changes are typically tabulated for reactions in which both
the reactants and products are at the same conditions. A standard state is a commonly accepted set of conditions used as a reference point
for the determination of properties under other different conditions. For
chemists, the IUPAC standard state refers to materials under a pressure of 1 bar
and solutions at 1 M, and does not specify a temperature. Many thermochemical
tables list values with a standard state of 1 atm. Because the ΔH of a reaction changes very little with such small changes in pressure
(1 bar = 0.987 atm), ΔH values (except for the most precisely measured values) are
essentially the same under both sets of standard conditions. We will include a
superscripted “o” in the enthalpy change symbol to designate standard state.
Since the usual (but not technically standard) temperature is 298.15 K, we will
use a subscripted “298” to designate this temperature. Thus, the symbol
(ΔH°298) is used to indicate an enthalpy change for a process occurring under
these conditions. (The symbol ΔH is used to indicate an enthalpy change for a reaction occurring under
nonstandard conditions) [21, p. 263].
Enthalpy of Combustion. Standard enthalpy of combustion (ΔHC°) is the enthalpy change when 1 mole of a substance burns (combines
vigorously with oxygen) under standard state conditions; it is sometimes
called “heat of combustion.” For example, the enthalpy of combustion of
ethanol, −1366.8 kJ/mol, is the amount of heat produced when one mole of
ethanol undergoes complete combustion at 25°C and 1 atmosphere pressure,
yielding products also at 25°C and 1 atm.
C2H5OH(l) + 3O2(g)⟶2CO2 + 3H2O(l) ΔH°298 = −1366.8 kJ
Enthalpies of combustion for many substances have been measured; a
few of these are listed in table 6.2. Many readily available substances with large enthalpies of
combustion are used as fuels, including hydrogen, carbon (as coal or charcoal),
and hydrocarbons (compounds containing only hydrogen and carbon), such as methane,
propane, and the major components of gasoline [2, p. 267].
Table 6.2
Standard Molar Enthalpies of Combustion
|
Substance |
Combustion
Reaction |
Enthalpy of Combustion, ΔHc° ( kJ/mol at 25 °C) |
|
Carbon |
C(s)
+ O2(g)
⟶
CO2(g) |
−393.5 |
|
Hydrogen |
H2(g)
+ 1/2O2(g)
⟶
H2O(l) |
−285.8 |
|
Magnesium |
Mg(s)
+ 1/2O2(g)
⟶
MgO(s) |
−601.6 |
|
Sulfur |
S(s)
+ O2(g)
⟶
SO2(g) |
−296.8 |
|
Carbon
monoxide |
CO(g)
+ 1/2O2(g)
⟶
CO2(g) |
−283.0 |
|
Methane |
CH4(g) + 2O2(g) ⟶ CO2(g) + 2H2O(l) |
−890.8 |
|
Acetylene |
C2H2(g) + 5/2O2(g) ⟶ 2CO2(g) + H2O(l) |
−1301.1 |
|
Ethanol |
C2H5OH(l) + 2O2(g) ⟶ CO2(g) + 3H2O(l) |
−1366.8 |
|
Methanol |
CH3OH(l) + 3/2O2(g) ⟶ CO2(g) + 2H2O(l) |
−726.1 |
|
Isooctane |
C8H18(l) + 25/2O2(g) ⟶ 8CO2(g) + 9H2O(l) |
−5461 |
Standard Enthalpy of Formation. A standard enthalpy of formation is an enthalpy change for a reaction in which exactly 1 mole of a
pure substance is formed from free elements in their most stable states under
standard state conditions. These values are especially useful for computing or
predicting enthalpy changes for chemical reactions that are impractical or
dangerous to carry out, or for processes for which it is difficult to make
measurements. If we have values for the appropriate standard enthalpies of
formation, we can determine the enthalpy change for any reaction, which we will
practice in the next section on Hess’s law.
The standard enthalpy of formation of CO2 (g) is −393.5 kJ/mol. This is the enthalpy change for the exothermic
reaction:
C(s) + O2 (g) ⟶ CO2 (g) ΔHf ° = ΔH°298 = −393.5 kJ
starting with the reactants at a pressure of 1 atm and 25 °C (with
the carbon present as graphite, the most stable form of carbon under these
conditions) and ending with one mole of CO2, also at 1 atm and 25 °C.
For nitrogen dioxide, NO2 (g), ΔHf ° is 33.2 kJ/mol. This is the enthalpy change for the
reaction:
1/2N2 (g) + O2 (g) ⟶ NO2 (g) ΔHf ° = ΔH°298 = +33.2 kJ
A reaction equation with ½ mole of N2 and 1 mole of
O2 is correct in this case because the standard enthalpy of formation
always refers to 1 mole of product, NO2(g).
You will find a
table of standard enthalpies of formation of many common substances in
Appendix G. These values indicate that formation reactions range from highly
exothermic (such as −2984 kJ/mol for the formation of P4O10) to strongly
endothermic (such as +226.7 kJ/mol for the formation of acetylene,
C2H2). By definition, the standard enthalpy of formation
of an element in its most stable form is equal to zero under standard
conditions, which is 1 atm for gases and 1 M for solutions [2, p.
271].
Hess’s Law. There are two ways to determine the amount of heat involved in a
chemical change: measure it experimentally, or calculate it from other
experimentally determined enthalpy changes. Some reactions are difficult, if not
impossible, to investigate and make accurate measurements for
experimentally. And even when a reaction is not hard to perform or
measure, it is convenient to be able to determine the heat involved in a
reaction without having to perform an experiment.
This type of calculation usually involves the use of Hess’s law, which states: If a process can be written as the sum of several stepwise processes,
the enthalpy change of the total process equals the sum of the enthalpy changes
of the various steps.
Hess’s law is valid because enthalpy is a state function: Enthalpy
changes depend only on where a chemical process starts and ends, but not on the path it takes from
start to finish. For example, we can think of the reaction of carbon with oxygen to form carbon dioxide as
occurring either directly or by a two-step process. The direct process is written:
C(s) + O2(g)⟶CO2(g) ΔH°298 = −394 kJ
In the two-step process, first carbon monoxide is
formed:
C(s) + 1/2O2(g)⟶CO(g) ΔH°298 = −111 kJ
Then, carbon monoxide reacts further to form carbon
dioxide:
CO(g) + 1/2O2(g)⟶CO(g) ΔH°298 = −283 kJ
The equation describing the overall reaction is the sum of these two
chemical changes:
Step 1: C(s) + 1/2O2(g) ⟶ CO(g)
Step 2: CO(g) + 1/2O2(g) ⟶ CO2(g)
Sum: C(s) + 1/2O2(g) + CO(g) + 1/2O2(g) ⟶ CO(g) + CO2(g)
Because the CO produced in Step 1 is consumed in Step 2, the net
change is:
C(s) + O2(g) ⟶ CO2(g)
According to Hess’s law, the enthalpy change of the reaction will
equal the sum of the enthalpy changes of the steps. We can apply the data from
the experimental enthalpies of combustion in table 6.2 to find the enthalpy
change of the entire reaction from its two steps:
C(s) + 1/2O2(g)⟶CO(g) ΔH°298 = −111 kJ
CO(g) + 1/2O2(g) ⟶ CO2(g) ΔH°298 = −283 kJ
C(s) + O2(g) ⟶ CO2(g) ΔH°298 = −394 kJ
The result is
shown in figure 6.8. We see that
ΔH of the overall reaction is the same whether it occurs in one step or
two. This finding (overall ΔH for the reaction = sum of ΔH values for reaction “steps” in the overall reaction) is true in
general for chemical and physical processes.
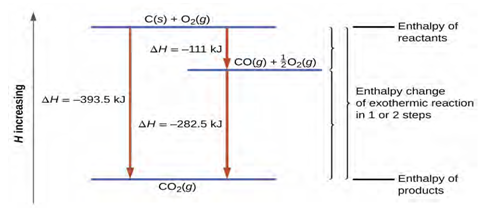
Figure 6.8 The formation of CO2 (g) from its elements can be thought of as occurring in two steps,
which sum to the overall reaction, as described by Hess’s law. The horizontal
blue lines represent enthalpies. For an exothermic process, the products are at
lower enthalpy than are the reactants.
Before we further practice using Hess’s law, let us recall two
important features of ΔH.
1.
ΔH is directly proportional to the quantities of reactants or products.
For example, the enthalpy change for the reaction forming 1 mole of
NO2(g) is +33.2 kJ:
1/2N2 (g) + O2 (g) ⟶ NO2 (g) ΔH = +33.2 kJ
When 2 moles of NO2 (twice as much) are formed, the
ΔH will be twice as large:
N2(g) + 2O2(g) ⟶ 2NO2(g) ΔH = +66.4 kJ
In general, if we multiply or divide an equation by a number, then
the enthalpy change should also be multiplied or divided by the same
number.
2.
ΔH for a reaction in one direction is equal in magnitude and opposite in
sign to ΔH for the reaction in the reverse direction. For example, given
that:
H2(g) + Cl2(g) ⟶ 2HCl(g) ΔH = −184.6 kJ
Then, for the “reverse” reaction, the enthalpy change is also
“reversed”:
2HCl(g) ⟶ H2(g) + Cl2(g) ΔH = +184.6 kJ
We also can use Hess’s law to determine the enthalpy change of any
reaction if the corresponding enthalpies of formation of the reactants and
products are available. The stepwise reactions we consider are: (i)
decompositions of the reactants into their component elements (for which the
enthalpy changes are proportional to the negative of the enthalpies of formation
of the reactants), followed by (ii) re-combinations of the elements to give the
products (with the enthalpy changes proportional to the enthalpies of formation
of the products). The standard enthalpy change of the overall reaction is
therefore equal to: (ii) the sum of the standard enthalpies of formation of all
the products plus (i) the sum of the negatives of the standard enthalpies of
formation of the reactants. This is usually rearranged slightly to be written as
follows, with Σ representing “the sum of” and n standing for the stoichiometric coefficients:
ΔHreaction ° =Σn × ΔHf ° (products) − Σ
n × ΔHf ° (reactants) [2, p. 273]
Gibbs Energy. This function has been introduced by Gibbs. The symbol of Gibbs energy is G. Its
significance and use are related to an
isothermal and isobaric process. More precisely, the Gibbs energy is related
to a process in which the
temperature and the pressure of the studied system remain equal to the temperature and pressure
of its surroundings (at least at the beginning and at the end of the process
provided, in this case, that during it, the surroundings remain at the constant temperature
Text and pressure pext):
p = pext and T = Text
The Gibbs energy is defined by the expression
(6)
G = U + pV - TS (6)
where U, p, V, T, and S are, respectively, the internal energy,
pressure, volume, temperature, and entropy of the system. Its unity is the Joule
J. Owing to its definition, the Gibbs energy is a state function.
The interest of the introduction of this function is the following
one: it turns out that the Gibbs function may constitute a criterion of
equilibrium and also of evolution specially convenient for any process at
constant pressure and temperature.
Let us, for example, study the process with the aid of which we want
to recover useful work (every work other than that stemming from the change in
the volume of the system), starting from the system. (A good example is that of
an electrochemical cell producing electrical energy which is connected to an
electrical motor. The cell has the property to transform the chemical
energy—coming from the two electrochemical reactions which simultaneously take
place at each of both electrodes—to electrical work.) It is demonstrated in an
absolute general manner that the work given to the surroundings is always weaker
than the change in internal energy of the system. In other words, the Gibbs
energy of the system cannot do anything else than to decrease when it supplies
work to the surroundings, in any case when the process is spontaneous. Hence, we
can deduce that
ΔG ≤ 0
or in differential writing
dG ≤ 0
At equilibrium
dG = 0
For a system at equilibrium at given pressure and temperature, the
Gibbs energy is at its minimum value.
Hence, with the introduction of the function of Gibbs energy, the
criterion of spontaneous evolution of a system, that is to say that of the
change in the total entropy (that of the system plus that of its
surroundings—both forming an isolated system), is transformed into another one
which is the criterion of the decrease of the Gibbs energy of the studied system
alone. The latter criterion is evidently less heavy than the former and is
easier to handle because it does not require the knowledge of the thermodynamic
parameters defining the state of the surroundings. However, the criterion of the
Gibbs energy is by far less general than that of entropy because, for its
handling, it implies that the process evolves at constant temperature and
pressure.
The Gibbs energy function can be defined in another way. Of course,
all its definitions are equivalent. For example, taking into account the fact
that the enthalpy of a system is defined by the expression
H = U + pV
the Gibbs energy can also be written (7)
G = H – TS (7)
or
dG = dH - TdS – SdT [22, p.3]