The rates of chemical reactions. Chemical equilibrium
The Rates of Chemical Reactions. Introduction. The objective of this chapter is to obtain an empirical description
of the rates of chemical reactions on a macroscopic level and to relate
the laws describing those rates to mechanisms for reaction on the
microscopic level. Experimentally, it is found that the rate of a
reaction depends on a variety of factors: on the temperature, pressure, and
volume of the reaction vessel; on the concentrations of the reactants and
products; on whether or not a catalyst is present. By observing how the
rate changes with such parameters, an intelligent chemist can learn what
might be happening at the molecular level. The goal, then, is to describe
in as much detail as possible the reaction mechanism. This goal is achieved in
several steps. First, in this chapter, we will learn how an overall
mechanism can be described in terms of a series of elementary
steps. In later chapters, we will continue our pursuit of a detailed
description 1) by examining how to predict and interpret values for the
rate constants in these elementary steps and 2) by examining how
the elementary steps might depend on the type and distribution of energy
among the available degrees of freedom. In addition to these lofty
intellectual pursuits, of course, there are very good practical reasons for
understanding how reactions take place, reasons ranging from the desire
for control of synthetic pathways to the need for understanding of the
chemistry of the earth's atmosphere [18, p. 3].
Empirical Observations: Measurement of Reaction Rates.
One of the most fundamental empirical observations that a chemist can
make is how the concentrations of reactants and products vary with time. The
first substantial quantitative study of the rate of a reaction was performed by
L. Wilhelmy, who in 1850 studied the inversion of sucrose in acid solution with
a polarimeter. There are many methods for making such observations: one might
monitor the concentrations spectroscopically, through absorption, fluorescence,
or light scattering; one might measure concentrations electrochemically, for
example, by potentiometric determination of the pH; one might monitor the total
volume or pressure if these are related in a simple way to the concentrations.
In general, as is true in this figure, the reactant concentrations
will decrease as time goes on, while the product concentrations will increase.
There may also be "intermediates" in the reaction, species whose concentrations
first grow and then decay with time [18, p.3].
Rates of Reactions, Differential and Integrated Rate Laws.
We define the rate law for a reaction in terms of the time
rate of change in concentration of one of the reactants or products. In general,
the rate of change of the chosen species will be a function of the
concentrations of the reactant and product species as well as of external
parameters such as the temperature. Rate of change for a species at any time is
proportional to the slope of its concentration curve. The slope varies with time
and generally approaches zero as the reaction approaches equilibrium. The
stoichiometry of the reaction determines the proportionality constant. Consider
the general reaction
aA + bB → cC + dD
We will define the rate of change of [C] as rate =
(1/c)d[C]/dt. This rate varies with time and is equal to some function of
the concentrations: (1/c)d[C]dt = f([A],[B],[C],[D]). Of course,
the time rates of change for the concentrations of the other species in the
reaction are related to that of the first species by the stoichiometry of the
reaction. For the example presented above, we find that
(1)
(1/c)(d[C]/dt) = (1/d)(d[D]/dt) = (1/a)(d[A]/dt) = (1/b)(d[B]/dt)
(1)
The equation (1/c)d[C]/dt = f([A],[B],[C],[D]) is
called the rate law for the reaction. While f([A],[B],[C],[D])
might in general be a complicated function of the concentrations, it often
occurs that f can be expressed as a simple product of a rate constant,
k, and the concentrations each raised to some power
(2):
(1/c)(d[C]/dt) =
k[A]m[B]n[C]o[D]p
(2)
When the rate law can be written in this simple way, we define the
overall order of the reaction as the sum of the powers, i.e., overall
order q = m+n+o+p, and we define the order of the reaction
with respect to a particular species as the power to which its concentration
is raised in the rate law, e.g., order with respect to [A] = m. Note that
since the left hand side of the above equation has units of concentration per
time, the rate constant will have units of time-1
concentration-(q-1). As we will see below, the form of the rate law
and the order with respect to each species give us a clue to the mechanism of
the reaction. In addition, of course,
the rate law allows us to predict how the concentrations of the
various species change with time.
An important distinction should be made from the outset: the overall
order of a reaction cannot be obtained simply by looking at the overall
reaction. For example, one might think (mistakenly) that the
reaction
H2 + Br2 → 2HBr
should be second order simply because the reaction consumes one
molecule of H2 and one molecule of Br2. In fact, the rate
law for this reaction is quite different:
(1/c)(d[HBr]/dt) =
k[H2][Br2]1/2
Thus the order of a reaction is not necessarily related to the
stoichiometry of the reaction; it can be determined only by
experiment.
One technique is called the method of initial slopes. If we
were to keep [Br2] fixed while monitoring how the initial rate of
[HBr] production depended on the H2 starting concentration,
[H2]0, we would find, for example, that if we doubled
[H2]0, the rate of HBr production would increase by a factor of two.
By contrast, were we to fix the starting concentration of H2 and monitor how the
initial rate of HBr appearance rate depended on the Br2 starting
concentration, [Br2]0, we would find that if we doubled
[Br2]0, the HBr production rate would increase not by a factor of
two, but only by a factor of 2. Experiments such as these would thus show the
reaction to be first order with respect to H2 and half order with
respect to Br2.
While the rate law in its differential form describes in the simplest
terms how the rate of the reaction depends on the concentrations, it will often
be useful to determine how the concentrations themselves vary in time. Of
course, if we know d[C]/dt, in principle we can find [C] as a function of
time by integration. In practice, the equations are sometimes complicated, but
it is useful to consider the differential and integrated rate laws for some of
the simpler and more common reaction orders [18, p.4].
Characteristics of order of a reaction
·
The magnitude of order of a reaction may be zero, or fractional or
integral values. For an elementary reaction, its order is never fractional since
it is a one step process.
·
Order of a reaction should be determined only by experiments. It
cannot be predicted interms of stoichiometry of reactants and
products.
·
Simple reactions possess low values of order like n = 0, 1, 2.
Reactions with order greater than or equal to 3.0 are called complex reactions.
Higher order reactions are rare.
·
Some reactions show fractional order depending on
rate.
·
Higher order reactions may be experimentally converted into simpler
order (pseudo) reactions by using excess concentrations of one or more reactants
[20, p. 8].
Zero order reactions
Here the rate doesn’t depend on the concentration. The rate is
constant.
Kinetic equation is: R = dC/dt =
k0,
where C is concentration in mol/liter, t is time
in
seconds; k0
is the rate constant.
[k0]= [C][t–1] =
[mol×liter–1∙ sec–1].
First-Order Reactions. Let us start by considering first-order reactions, A products, for which the differential
form of the rate law is (3)
-d[A]/dt = k[A] (3)
Rearrangement of
this equation yields (4)
d[A]/[A] = -k dt (4)
Let [A(0)] be the initial concentration of A and let [A(t)] be
the concentration at time t. Then integration yields
(5)
ln[A(t)]/[A(0)] = -kt (6)
or, exponentiating both sides of the equation,
[A(t)] = [A(0)] exp(-kt) [18, p. 6]
Second-Order Reactions. Second-order reactions are of two types, those that are second order
in a single reactant and those that are first order in each of two reactants.
Consider first the former case, for which the simplest overall reaction
is
2 A → products
with the differential rate lawb
Rate = k [A]2
Of course, a simple method for obtaining the integrated rate law
would be to rearrange the differential law as (7)
-d[A]/[A]2 = kdt (7)
and to integrate from t=0 when [A]=[A(0)] to the final time
when [A]=[A(t)] (8).
1/[A(t)] – 1/[A(0)] = kt (8)
However, in order to prepare the way for more complicated
integrations, it is useful to perform the integration another way by introducing
a change of variable. Let x be defined as the amount of A that has
reacted at any given time. Then [A(t)] =
[A(0)]-x.
Rate of reaction. The rate of reaction i.e. the velocity of a reaction is the amount of
a chemical change occurring per unit time.
The rate is generally expressed as the decrease in concentration of a
reactant or as the increase in concentration of the product. If C is the
concentration of a reactant at any time t, the rate is – dC/dt or if the
concentration of a product be x at
any time t, the rate would be dC/dt.
The unit of reaction rate is
moles/litre/second.
Factors influencing the rate of reaction. Rate of a chemical reaction is influenced by the following
factors
·
Temperature. In most cases, the rate of a reaction in a homogeneous
reaction is approximately doubled or tripled by an increase in temperature of
only 100 C. In some cases the rise in reaction rates are even
higher.
·
Concentration of the reactants. At a fixed temperature and in the
absence of catalyst, the rate of given reaction increases with increased
concentration of reactants. With increasing concentration of the reactant the
number of molecules per unit volume is increased, thus the collision frequency
is increased, which ultimately causes increased reaction
rate.
·
Nature of reactants. A chemical reaction involves the rearrangement
of atoms between the reacting molecules to the product. Old bonds are broken and
new bonds are formed. Consequently, the nature and the strength of the bonds in
reactant molecules greatly influence the rate of its transformation into
products. The reaction in which involve lesser bond rearrangement proceeds much
faster than those which involve larger bond rearrangement.
·
Catalysts. The rate of a chemical reaction is increased in presence
of a catalyst which ultimately enhanced the speed of a chemical
reaction.
·
Radiation The rate of a number of chemical reactions increases when
radiations of specific wave length are absorbed by the reacting molecules. Such
reactions are called photochemical reactions. For example, chlorine may be mixed
safely with hydrogen in dark, since the reaction between the two is very slow.
However when the mixture is exposed to light, the reaction is
explosive.
H2 + Cl2→ 2HCl + 188KJ
Molecularity. It is defined as the number of molecules colliding and leading to
chemical transformations. Molecularity characterizes the simple reaction, i.e.
elementary act of the reaction (individual steps by which a reaction
proceeds).Molecularity has a definite physical
sense.
Classification of molecularity:
1.
Unimolecular reactions are some molecular decompositions and intramolecular
rearrangements:
CH3NH2 → HCN +
2H2
CaCO3 → CaO + CO2
2.
Bimolecular reactions are those resulting from collision of two molecules of the same or
different species:
CO + Cl2 → COCl2
I2 + H2 → 2HI
3.
Trimolecular reactions are those which require a collision between three molecules:
2NO + H2 = N2O +
H2O
Practically no reactions of a higher molecularity are
known.
Molecularity is always a whole number and never greater than three.
A molecularity of four is not known because collision of four particles in a
single step is not
favorable. When the
equation of the reaction indicates that a large number of molecules
participate, this usually means
that the process must proceed in a more
complicated manner, namely through two or more consecutive stages of which each is
due to collision between two,
or, rarely, three molecules.
For example,
3Н2 + N2 → 2NH3
It is a complex reaction.
For simple
reactions the order of reaction and molecularity coincides. For complex
reactions the order of reaction and molecularity doesn’t coincidemore
off.
H2O2 + 2HI → I2 +
2H2O
First step: H2O2 + HI → HOI + H2O
slow
Second step: HIO + HI → I2 + H2O
fast
General rate of this reaction is determined by the slowest step,
which is called the rate controlling or rate determining step. The seeming molecularity
of this reaction is three. This reaction
is complex; the order of this reaction is two. It is known,
if molecularity and the order don’t coincide, it means:
1.
the reaction is
complex,
2.
the rate of this reaction is limited by rate determining step.
Chemical equilibrium. When a chemical reaction takes place in a container which prevents
the entry or escape of any of the substances involved in the reaction,
the quantities of these components change as some are consumed and others
are formed. Eventually this change will come to an end, after which the
composition will remain unchanged as long as the system remains undisturbed.
The system is then said to be in its equilibrium state, or more simply,
“at equilibrium”.
The direction in
which we write a chemical reaction (and thus which components are considered
reactants and which are products) is arbitrary. Thus the
equations
H2 + I2 → 2HI ”synthesis of hydrogen
iodide”
and
2HI → H2 + I2 ”dissociation of hydrogen
iodide”
represent the same chemical reaction system in which the roles of the
components are reversed, and both
yield the same mixture of components when the change is
completed.
This last point is central to the concept of chemical equilibrium. It
makes no difference whether we start with two moles of HI or one mole each of
H2 and I2; once the reaction has run to completion, the
quantities of these two components will be the same. In general, then, we can
say that the composition of a chemical reaction system will tend to change in a
direction that brings it closer to its equilibrium composition. Once this
equilibrium composition has been attained, no further change in the quantities
of the components will occur as long as the system remains undisturbed [19,
p.2].
Reversible reaction. A chemical equation of the form A → B represents the transformation
of A into B, but it does not imply that all of the reactants will be
converted into products, or that the reverse reaction B → A cannot also occur.
In general, both processes can be expected to occur, resulting in an
equilibrium mixture containing all of the components of the reaction
system. (We use the word components when we do not wish to distinguish
between reactants and products.) If the equilibrium state is one in which
significant quantities of both reactants and products are present (as in the
hydrogen iodide example given above), then the reaction is said to incomplete
or reversible.
The latter term is preferable because it avoids confusion with
“complete” in its other sense of being finished, implying that the reaction has
run its course and is now at equilibrium.
·
If it is desired to emphasize the reversibility of a reaction, the
single arrow in the equation is replaced with a pair of hooked lines pointing in
opposite directions, as in A B. There is no fundamental difference between the
meanings of A → B and A B, however. Some older textbooks even use A =
B.
·
A reaction is said to be complete when the equilibrium
composition contains no significant amount of the reactants. However, a reaction
that is complete when written in one direction is said “not to occur” when
written in the reverse direction.
In principle, all chemical reactions are reversible, but this
reversibility may not be observable if the fraction of products in the
equilibrium mixture is very small, or if the reverse reaction is kinetically
inhibited [19, p.4].
The Law of Mass Action. Berthollet’s ideas about reversible reactions were finally vindicated
by experiments carried out by others, most notably the Norwegian chemists (and
brothers-in-law) Cato Guldberg and Peter Waage. During the period 1864-1879 they
showed that an equilibrium can be approached from either direction (see the
hydrogen iodide illustration above), implying that any reaction aA +
bB → cC + dD is really a competition between a “forward”
and a “reverse” reaction. When a reaction is at equilibrium, the rates of these
two reactions are identical, so no net (macroscopic) change is observed,
although individual components are actively being transformed at the microscopic
level.
Guldberg and Waage showed that the rate of the reaction in either
direction is proportional to what they called the “active masses” of the various
components:
rate of forward reaction =
kf[A]a[B]b
rate of reverse reaction =
kr[C]c[D]d
in which the proportionality constants k are called rate
constants and the quantities in square brackets represent concentrations. If
we combine the two reactants A and B, the forward reaction starts immediately,
but the formation of products allows the reverse process to get underway. As the
reaction proceeds, the rate of the forward reaction diminishes while that of the
reverse reaction increases. Eventually the two processes are proceeding at the
same rate, and the reaction is at equilibrium (9):
rate of forward reaction = rate of reverse
reaction
kf[A]a[B]b =
kr[C]c[D]d (9)
If we now change the composition of the system by adding some C or
withdrawing some A (thus changing their “active masses”), the reverse rate will
exceed the forward rate and a change in composition will occur until a new
equilibrium composition is achieved.
The Law of Mass Action is thus essentially the statement that the
equilibrium composition of a reaction mixture can vary according to the
quantities of components that are present. This of course is just what
Berthollet observed in his Egyptian salt ponds, but it was now seen to be a
consequence of the dynamic nature of chemical equilibrium [19,
p.5].
The LeChâtelier principle. If a reaction is at equilibrium and we alter the conditions so as to
create a new equilibrium state, then the composition of the system will tend to
change until that new equilibrium state is attained. (We say “tend to
change” because if the reaction is kinetically inhibited, the change may be too
slow to observe or it may never take place.) In 1884, the French chemical
engineer and teacher Henri LeChâtelier (1850-1936) showed that in every such
case, the new equilibrium state is one that partially reduces the effect of the
change that brought it about. This law is known to every Chemistry student as
the LeChâtelier principle. His original formulation was somewhat more
complicated, but a reasonably useful paraphrase of it reads as
follows:
LeChâtelier principle: If a system at equilibrium is subjected to a change of pressure,
temperature, or the number of moles of a substance, there will be a tendency for
a net reaction in the direction that tends to reduce the effect of this
change.
To see how this works (and you must do so, as this is of such
fundamental importance that you simply cannot do any meaningful Chemistry
without a thorough working understanding of this principle), look again the
hydrogen iodide dissociation reaction
2HI → H2 + I2
Consider an arbitrary mixture of these components at equilibrium, and
assume that we inject more hydrogen gas into the container. Because the
H2 concentration now exceeds its new equilibrium value, the system is
no longer in its equilibrium state, so a net reaction now ensues as the system
moves to the new state. The LeChâtelier principle states that the net reaction
will be in a direction that tends to reduce the effect of the added
H2. This can occur if some of the H2 is consumed by
reacting with I2 to form more HI; in other words, a net reaction
occurs in the reverse direction. Chemists usually simply say that “the
equilibrium shifts to the left”.
To get a better idea of how this works, carefully examine the diagram
below which followsthe concentrations of the three components of this reaction
as they might change in time (the time scale here will typically be about an
hour).The following table contains several examples showing how changing the
quantity of a reaction component can shift an established equilibrium The
following table contains several examples showing how changing the quantity of a
reaction component can shift an established equilibrium [19,
p.8].
The LeChâtelier principle in physiology: hemoglobin and oxygen
transport. Many of the chemical reactions that occur in living organisms are
regulated through the LeChâtelier principle. Few of these are more important to
warm-blooded organisms than those that relate to aerobic respiration, in which
oxygen is transported to the cells where it is combined with glucose and
metabolized to carbon dioxide, which then moves back to the lungs from which it
is expelled.
hemoglobin + O2 oxyhemoglobin
The partial pressure of O2 in the air is 0.2 atm,
sufficient to allow these molecules to be taken up by hemoglobin (the red
pigment of blood) in which it becomes loosely bound in a complex known as
oxyhemoglobin. At the ends of the capillaries which deliver the blood to the
tissues, the O2 concentration is reduced by about 50% owing to its
consumption by the
cells. This shifts the equilibrium to the left, releasing the oxygen
so it can diffuse into the cells.
Carbon dioxide reacts with water to form the weak acid
H2CO3 which would cause the blood acidity to become
dangerously high if it were not promptly removed as it is excreted by the cells.
This is accomplished by combining it with carbonate ion through the
reaction
H2CO3 + CO3-2 ↔
2HCO3-
which is forced to the right by the high local CO2
concentration within the tissues. Once the hydrogen carbonate (bicarbonate) ions
reach the lung tissues where the CO2 partial pressure is much
smaller, the reaction reverses and the CO2 is expelled [19, p.
11].
Predicting the Direction of a Reversible Reaction. Le Chatelier's principle can be used to predict changes in
equilibrium concentrations when a system that is at equilibrium is subjected to
a stress. However, if we have a mixture of reactants and products that have not
yet reached equilibrium, the changes necessary to reach equilibrium may not be
so obvious. In such a case, we can compare the values of Q and K for the system to predict the changes [2, p.
741].
Effect of Change in Concentration on Equilibrium. A chemical system at equilibrium can be temporarily shifted out of
equilibrium by adding or removing one or more of the reactants or
products. The concentrations of both reactants and products then undergo
additional changes to return the system to equilibrium.
The stress on the system in figure 5.1 is the reduction of
the equilibrium concentration of SCN− (lowering the
concentration of one of the reactants would cause Q to be larger than K). As a consequence, Le Chatelier's principle leads us to
predict that the concentration of Fe(SCN)2+ should decrease,
increasing the concentration of SCN− part way back to its
original concentration, and increasing the concentration of Fe3+
above its initial equilibrium concentration.
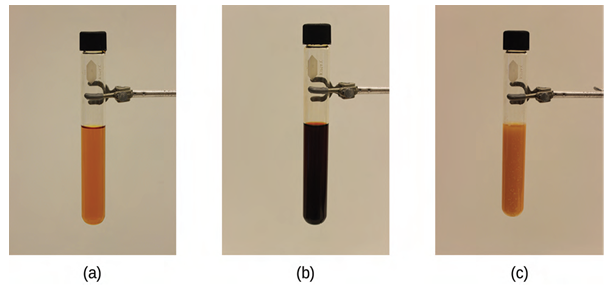
Figure 5.1 (a) The test tube contains 0.1 M Fe3+. (b) Thiocyanate ion has been added to solution in
(a), forming the red Fe(SCN)2+ ion. Fe3+(aq) + SCN−(aq) ↔ Fe(SCN)2+(aq). (c) Silver nitrate has been added to the solution in (b),
precipitating some of the SCN− as the white solid AgSCN. Ag+(aq) + SCN−(aq) ↔ AgSCN(s). The decrease in the SCN− concentration shifts the first
equilibrium in the solution to the left, decreasing the concentration (and
lightening color) of the Fe(SCN)2+.
The effect of a change in concentration on a system at equilibrium is
illustrated further by the equilibrium of this chemical
reaction:
H2 (g) + I2 (g) ↔ 2HI (g) ; Kc = 50.0 at 400 °C
The numeric values for this example have been determined
experimentally. A mixture of gases at 400 °C with [H2] =
[I2] = 0.221 M and [HI] = 1.563 M is at equilibrium; for this mixture, Qc = Kc = 50.0. If H2 is introduced into the system so quickly that its
concentration doubles before it begins to react (new [H2] = 0.442
M), the reaction will shift so that a new equilibrium is reached, at
which [H2] = 0.374 M, [I2] = 0.153 M, and [HI] = 1.692 M. This gives:
Qc = [HI]2/[H2][I2] =
(1,692)2/(0,374)(0,153) = 50,0 = Kc
We have stressed this system by introducing additional H2.
The stress is relieved when the reaction shifts to the right, using up some (but
not all) of the excess H2, reducing the amount of uncombined
I2, and forming additional HI [2, p. 741].
Effect of Change in Pressure on Equilibrium. Sometimes we can change the position of equilibrium by changing the
pressure of a system. However, changes in pressure have a measurable
effect only in systems in which gases are involved, and then only when the
chemical reaction produces a change in the total number of gas molecules
in the system. An easy way to recognize such a system is to look for
different numbers of moles of gas on the reactant and product sides of the
equilibrium. While evaluating pressure (as well as related factors like
volume), it is important to remember that equilibrium constants are
defined with regard to concentration (for Kc) or partial pressure (for KP). Some changes to total pressure, like adding an inert gas that is
not part of the equilibrium, will change the total pressure but not the partial
pressures of the gases in the equilibrium constant expression. Thus, addition of
a gas not involved in the equilibrium will not perturb the
equilibrium.
As we increase
the pressure of a gaseous system at equilibrium, either by decreasing the volume
of the system or by adding more of one of the components of the equilibrium
mixture, we introduce a stress by increasing the partial pressures of one or
more of the components. In accordance with Le Chatelier's principle, a shift in
the equilibrium that reduces the total number of molecules per unit of volume
will be favored because this relieves the stress. The reverse reaction would be
favored by a decrease in pressure.
Consider what happens when we increase the pressure on a system in
which NO, O2, and NO2 are at
equilibrium:
2NO (g) + O2 (g) → 2NO2 (g)
The formation of additional amounts of NO2 decreases the
total number of molecules in the system because each time two molecules of
NO2 form, a total of three molecules of NO and O2 are
consumed. This reduces the total pressure exerted by the system and reduces, but
does not completely relieve, the stress of the increased pressure. On the other
hand, a decrease in the pressure on the system favors decomposition of
NO2 into NO and O2, which tends to restore the
pressure.
Now consider this reaction:
N2 (g) + O2 (g) → 2NO (g)
Because there is no change in the total number of molecules in the
system during reaction, a change in pressure does not favor either formation or
decomposition of gaseous nitrogen monoxide [2, p. 742].