Chemical bonding
Introduction. Though the periodic table has a place for 118 elements, there are
obviously more substances in nature than 118 pure elements. This is
because atoms of elements can react with one another to form new
substances called compounds. When two or more elements combine, the
resulting compound is unique both chemically and physically from its
parent atoms. For example, sodium is a silver coloured metal that reacts so
violently with water that flames are produced when sodium gets wet. The
element chlorine is greenish coloured gas that is so poisonous that it
was used as a weapon in world war I. When chemically bonded together,
these two dangerous substances form the compound sodium chloride,
a compound so safe that we eat it every day – common table salt [16, p.
1].
Formation of a chemical bond. Free atoms of elements are in random motion and possess some energy.
Farther the atoms are, greater is their energy and lesser is the
stability. Two or more atoms unite to form a molecule because in doing
so, the energy of the united atoms is lowered. Thus the ‘molecule’
becomes stable in comparison to separate atoms. In other words, a stable
chemical union called ‘bond’ between two or more atoms comes into existence
only if the energy is lowered when the atoms come in close vicinity. The
lower the energy of the molecule, the stronger the bond and more is the
stability to the bonded atoms [16, p. 1].
Nature of chemical bond. A chemical bond is an attraction between atoms. It is the attraction
caused by the electromagnetic force between opposing charges either
between electrons and nuclei or as the result of a dipole attraction.
Since opposite charges attract via a simple electromagnetic force, the
negatively charged electrons revolving round the nucleus and the
positively charged protons in the nucleus attract each other. Also an
electron positioned between two nuclei will be attracted to both of them.
Thus, the most stable configuration of nuclei and electrons is one in
which the electrons spend more time between nuclei than anywhere else in
space. These electrons cause the nuclei to be attracted to each other and
this attraction results in the bond. Electrons occupy large volume compared to
the nuclei and this volume keeps the atomic nuclei relatively far apart as
compared with the size of the nuclei themselves.
The force of attraction which holds the two atoms together in a
molecule is called a chemical bond [16, p. 1].
What is a molecule? All atoms attract one another at small distances; the
universal attractive interactions known as van der Waals forces exist
between all matter, and play an important part in determining the properties of
liquids and solids. These attractions are extremely weak, however, and they lack
specificity: they do not lead to aggregates having any special structure or
composition. Chemical bonding connotes the existence of an aggregate of atoms
that is sufficiently stable to possess a characteristic structure and
composition. The important thing to understand about the definition written at
the left is that it is essentially an operational one; as our ability to
observe the characteristic properties of loosely-bound aggregates of atoms
increases, our ideas of what constitutes a molecule will change. This was
illustrated quite vividly in the early 1980’s, when metal clusters—
stable arrangements of 5-20 metallic atoms— were first characterized. These
had not previously been recognized as molecules because no one know how
to observe them. More recently, advances in technology that allow
chemists to observe chemical species that can only exist for tiny fractions of a
second have greatly extended the range of what we can call “molecules” [17, p.
4].
Stability and reactivity. The fall in energy when atoms join together is a measure of the
stability of the new aggregate. In order to be regarded as a
molecule, the aggregate must be sufficiently stable to
resist disruption by thermal motions long enough to enable the observation of
whatever distinctive properties and composition it might have. Some
molecules are stable or observable only under certain conditions: many, such
as KrF2, are so weakly bound that they decompose at all but
the lowest temperatures. Others, such as gaseous LiF, can be observed
only at temperatures around 1000 °C. There are many molecules that are
energetically stable, but are so reactive that their lifetimes are
too brief to make their observation possible. The molecule CH3,
methyl, is a good example: it can be formed by electrical discharge in
gaseous CH4, but it is so reactive that it reacts with almost
any molecule it strikes within a few collisions. It was not until the
development of spectroscopic methods (in which a molecule is characterized
by the wavelengths of light that it absorbs) that methyl was recognized as a
stable molecule that is an important intermediate in many chemical
processes ranging from flames to atmospheric chemistry [17, p. 4].
Observable properties of chemical bonds. Chemical bonds, of course, cannot be observed directly; the best we
can do is to carry out experiments on substances containing the
appropriate pair of atoms, and then try to make inferences about the
nature of the bonding force between them.
It is important to bear in mind that the exact properties of a
specific kind of bond will be determined in part by the nature of the
other bonds in the molecule; thus the energy and length of the C–H bond
will be somewhat dependent on what other atoms are connected to the
carbon atom. Similarly, the C-H bond length can vary by as much a 4
percent between different molecules. For this reason, the values listed in
tables of bond energy and bond length are usually averages taken
over a variety of environments for a specific atom
pair.
In some cases, such as C—O and C—C, the variations can be much
greater, approaching 20 percent. In these cases, the values fall into groups
which we interpret as representative of single- and multiple
bonds: double, and triple [17, p. 6].
Bond energies. The bond energy is the amount of work that must be done to pull two
atoms completely apart; in other words, it is the same as the depth of
the “well” in the potential energy. This is almost, but not quite the same as
the bond dissociation energy actually required to break the chemical
bond; the difference is the very small zero-point
energy.
Bond energies are
usually determined indirectly from thermodynamic data, but there are two main
experimental ways of measuring them directly:
1.
The direct thermochemical method involves separating the two
atoms by an electrical discharge or some other means, and then measuring the
heat given off when they recombine. Thus the energy of the C—C single bond can
be estimated from the heat of the recombination reaction between methyl
radicals, yielding ethane:
CH3 + CH3 →
H3CCH3
Although this method is simple in principle, it is not easy to carry
out experimentally. Thehighly reactive components must be prepared in high
purity and in a stream of moving gas.
2.
The spectroscopic method is based on the principle that
absorption of light whose wavelength corresponds to the bond energy will often
lead to the breaking of the bond and dissociation of the molecule. For some
bonds, this light falls into the green and blue regions of the spectrum, but for
most bonds ultraviolet light is required. The experiment is carried out by
observing the absorption of light by the substance being studied as the
wavelength is decreased. When the wavelength is sufficiently small to break the
bond, a characteristic change in the absorption pattern is
observed.
Spectroscopy is quite easily carried out and can yield highly precise
results, but this method is only applicable to a relatively small number of
simple molecules. The major problem is that the light must first be absorbed
by the molecule, and relatively few molecules happen to absorb light of a
wavelength that corresponds energetically to a bond
energy.
Experiments carried out on diatomic molecules such as O2
and CS yield unambiguous values of bond energy, but for more complex molecules
there are complications. For example, the heat given off in the CH3
combination reaction written above will also include a small component that
represents the differences in the energies of the C-H bonds in methyl and in
ethane. These can be corrected for by experimental data on reactions such
as
CH3 + H → CH4
CH2 + H → CH3
By assembling a large amount of experimental information of this
kind, a consistent set of average bond energies can be obtained. The energies of
double bonds are greater than those of single bonds, and those of triple bonds
are higher still [17, p. 6].
Use of bond energies in estimating heats of reaction.
One can often get a very good idea of how much heat will be absorbed
or given off in a reaction by simply finding the difference in the total
bond energies contained in the reactants and
products.
As an example, consider the reaction of chlorine with methane to
produce dichloromethane and hydrogen chloride:
CH4(g) + 2Cl2(g) → CHCl2(g) +
2HCl(g)
In this reaction, two C–H bonds and two Cl–Cl bonds are broken, and
two new C–H and H–Cl bonds are formed. The net change is
2(C–H) + 2(Cl–Cl) – 2(C–Cl) –2 (H–Cl) = (830 + 486 -660 - 864)
kJ
which comes to –208 kJ per mole of methane; this agrees quite well
with the observed heat of reaction, which is –202 kJ/mol [17, p.
7].
Bond lengths. The bond length is the internuclear distance: the distance between
the centers of the two bonded atoms. Bond distances are customarily
expressed in Angstrom units (1Å = 10-8 cm = 100 pm) and are mostly in the
range 1-2 Å. Even though the bond is vibrating, equilibrium bond lengths
can be determined to within 0.01Å.
Bond lengths
reflect the sizes of the atoms; thus those involving hydrogen can be
quite short. The shortest, H–H, is only 0.74Å. Multiply-bonded atoms are
closer together than singly-bonded ones; this is a major criterion for
experimentally determining the multiplicity of a
bond.
The most common
method of measuring bond lengths in solids is by analysis of the diffraction or
scattering of X-rays when they pass through the regularly-spaced atoms in the
crystal. For gaseous molecules, neutron- or electron-diffraction can also be
used [17, p. 8].
Stretching frequency and infrared absorption. When an atom is displaced from its equilibrium position in a
molecule, it is subject to a restoring force which increases with the
displacement. A spring follows the same law (Hooke’s law); a chemical
bond is therefore formally similar to a spring that has weights (atoms)
attached to its two ends. A mechanical system of this kind possesses a
natural vibrational frequency which depends on the masses of the weights
and the stiffness of the spring.
For ordinary chemical bonds, these natural frequencies correspond to
those of infrared light. Each wavelength of infrared light that excites the
vibrational motion of a particular bond will be absorbed by the molecule. In
general, the stronger the bond and the lighter the atoms it connects, the higher
will be its natural stretching frequency and the shorter the wavelength of light
absorbed by it. Thus the C–H bond absorbs at a shorter wavelength than does the
C–C bond, and C–C bonds are easily distinguished from C=C double bonds. Studies
on a wide variety of molecules have made it possible to determine the
wavelengths absorbed by each kind of bond. By plotting the degree of absorption
as a function of wavelength, one obtains the infrared spectrum of the
molecule which allows one to “see” what kinds of bonds are
present.
Actual infrared spectra are complicated by the presence of more
complex motions (stretches involving more than two atoms, wagging, etc.), and
absorption to higher quantum states (overtones), so infrared spectra can become
quite complex. This is not necessarily a disadvantage, however, because such
spectra can serve as a “fingerprint” that is unique to a particular molecule and
can be helpful in identifying it. Largely for this reason, infrared
spectrometers are standard equipment in most chemistry laboratories [17, p.
8].
Lewis theory. In 1916, an American chemist, Lewis proposed that chemical bonds are
formed between atoms because electrons from the atoms interact with each
other. Lewis had observed that many elements are most stable when they
contain eight electrons in their outermost or valence shell of the atom.
He suggested that atoms with fewer than eight electrons bond together to
share electrons and complete their valence shell.
While some of Lewis predictions have since been proven incorrect (he
suggested that electrons occupy cube – shaped orbitals), his work
established the basis of what is known today about chemical bonding [16,
p. 2].
Essentials of Lewis theory. Between 1916 and 1919, Lewis, Kossel and Langmuir made several
important proposals on bonding which lead to the development of Lewis
theory of bonding.
1.
Valence electrons mainly play a fundamental role in
bonding.
2.
Ionic bonding involves the transfer of one or more electrons from one
atom to another.
3.
Covalent bonding involves sharing of electrons between
atoms.
4.
Electrons are transferred or shared between atoms such that each atom
achieves the electron configuration of a noble gas i.e. having eight electrons
in the outermost shell called octet.
5.
This arrangement is called octet rule. ( Exception –
He)
6.
Exceptions to octet rule may occur.
Lewis proposed symbols which represent the resulting structures that
follow the octet rule. In a Lewis symbol, an element is surrounded by up to 8
dots where elemental symbol represents the nucleus and the dots represents the
valence electrons [16, p. 2].
Covalent bond. Formation of covalent bond. The second major type of chemical bond occurs when atoms share
electrons. As opposed to ionic bonding in which a complete transfer of
electrons occurs, covalent bonding occurs when two ( or more ) elements
share electrons. Covalent bonding occurs because the atoms in the
molecule have a similar tendency for electrons ( generally to gain
electrons.) This most commonly occurs when two non-metals bond together. Because
both of the non-metals want to gain electrons , the elements involved will share
electrons in an effort to fill their valence shells. A good example of a
covalent bond is that which occurs between two hydrogen atoms. Atoms of hydrogen
(H) have one valence electron in their electron shell. Since the capacity of
this shell is two electrons, each hydrogen atom will ‘want’ to pick up a second
electron. In an effort to pick up a second electron, hydrogen atoms will react
with nearby hydrogen (H) atoms to form the molecule H2. Since the
hydrogen molecule is a combination of equally matched atoms, the atoms
will share each other’s single electron, forming one covalent bond. In this way,
both atoms share the stability of a full valence
shell.
A chemical bond formed by sharing of electrons between atoms is
called a covalent bond.
As the two hydrogen atoms approach one another, in addition to
nucleus – electron attraction, nuclear-nuclear repulsion and electron – electron
repulsion also come into existence. When the two hydrogen atoms are at a
distance of 0.074 nm, the potential energy of the two hydrogen atoms together is
at its minimum and releases 4.52 eV. At this stage, a chemical bond is formed.
If the hydrogen atoms come still closer, the potential energy rises steeply
making the molecule unstable. Thus, the sharing of electrons is energetically
favourable to both the hydrogen atoms with the formation of stable single
covalent bond.
The figure 4.1 given below shows the variation of potential energy as
a function of distance of separation of hydrogen atoms.
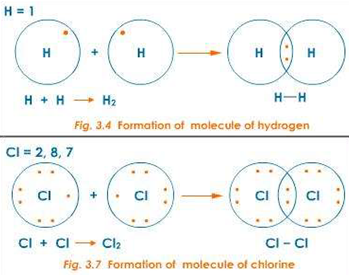
Figure 4.1 Bonding in H2 and Cl2
molecule
Two or more atoms of different elements can also share electrons to
form a single bond between them and complete the octet (or duplet) of each atom.
For example, in methane, one carbon and four hydrogen atoms share one electron
pair each to form four C – H bonds, in ammonia, one nitrogen and three hydrogen
atoms share one electron pair each to form three N – H bonds and in water, one
oxygen and two hydrogen atoms share one electron pair each to form two O – H
bonds. This is shown in the following diagram [16, p. 10].
Multiple bonds. For every pair of electrons shared between two atoms, a single
covalent bond is
formed. Some atoms can share two or three pairs of electrons forming
multiple bonds i. e. a double or triple bonds. For example, oxygen atom has six
electrons in its outermost shell. It needs two electrons to complete its octet
and attains the configuration of neon.
Hence two oxygen atoms combine by sharing two pairs of electrons
between them and form a double bond. Similarly, nitrogen atom has five electrons
in its outermost shell. It needs three electrons to complete its octet and
attain the configuration of the inert gas neon. Hence, two nitrogen atoms
combine by sharing three pairs of electrons between them and form a triple bond.
In HCN molecule, H and C atoms share one pair of electron to form a single bond
while C and N atoms share three pairs of electrons to form a triple
bond.
Following figure 4.2
shows the multiple bonds in O2, N2 and HCN molecules. [16, p.
12]
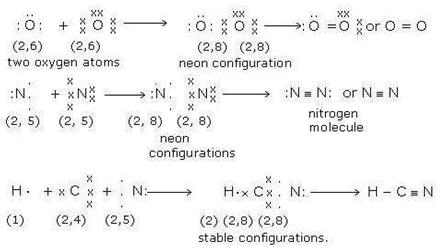
Figure 4.2 Bonding in O2, N2 and HCN molecules.
Polar and nonpolar bonds. Electronegativity. The electrons constituting a chemical bond are simultaneously
attracted by the electrostatic fields of the nuclei of the two bonded
atoms. In a homonuclear molecule such as O2 the bonding
electrons will be shared equally by the two atoms. In general, however,
differences in the sizes and nuclear charges of the atoms will cause one of
them to exert a greater attraction on the bonding pair, causing the
electron cloud to be displaced toward the more strongly-attracting
atom.
The electronegativity of an atom denotes its relative
electron-attracting power in a chemical bond.
It is important to understand that electronegativity is not a
measurable property of an atom in the sense that ionization energies and
electron affinities are, although it can be correlated with both of these
properties. The actual electron-attracting power of an atom depends in part on
its chemical environment (that is, on what other atoms are bonded to it), so
tabulated electronegativities should be regarded as high-precision predictors of
the behavior of electrons in more complicated molecules.
There are several ways of computing electronegativities, which are
expressed on an arbitrary scale. The concept of electronegativity was introduced
by Linus Pauling and his 0-4 scale continues to be the one most widely used [17,
p. 23].
Dipole moments. When non-identical atoms are joined in a covalent bond, the electron
pair will be attracted more strongly to the atom that has the higher
electronegativity. As a consequence, the electrons will not be shared equally;
the center of the negative charges in the molecule will be displaced from the
center of positive charge. Such bonds are said to be polar and to possess
partial ionic character, and they may confer a polar nature on the
molecule as a whole. A polar molecule acts as an electric dipole which
can interact with electric fields that are created artificially or that arise
from nearby ions or polar molecules. Dipoles are conventionally represented as
arrows pointing in the direction of the negative end. The magnitude of
interaction with the electric field is given by the permanent electric
dipole moment of the molecule. The dipole moment corresponding to an
individual bond (or to a diatomic molecule) is given by the product of the
quantity of charge displaced q = δ± and the bond length r (1):
μ
= q × r (1)
In SI units, q is expressed in coulombs and r in meters, so m
has the dimensions of Cm. If one entire electron charge is displaced by 100 pm
(a typical bond length), then
m = (1.6022 × 10–19 C) × (10–10 m) = 1.6 ×
10–29 C-m = 4.8 D
The quantity at the right, the Debye unit, is still commonly
used to express dipole moments. It was named after Peter Debye (1884-1966), the
Dutch physicist who pioneered the study of dipole moments and of electrical
interactions between particles, and won the Nobel Prize for Chemistry in 1934
[17, p. 23].
Measurement of dipole moments. When a solution of polar molecules is placed between two
oppositely-charged plates, they will tend to align themselves along the
direction of the field. This process consumes energy which is returned to the
electrical circuit when the field is switched off, an effect known as
electrical capacitance. Measurement of the capacitance of a gas or
solution is easy to carry out and serves as a means of determining the magnitude
of the dipole moment of a substance (figure 4.3).
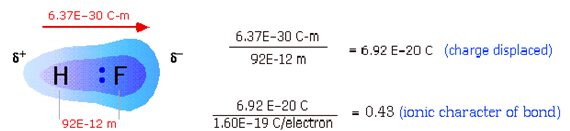
Figure 4.3 Dipole moment of HF
Dipole moments of more complicated molecules. In molecules containing more than one polar bond, the molecular
dipole moment is just the vector combination of the individual bond
dipoles. In some cases this can result in a molecule containing polar
bonds to be nonpolar, as in the example of carbon dioxide shown in figure
4.4. In molecules containing nonbonding electrons or multiple bonds, the
elecronegativity difference may not correctly predict the bond polarity. A
good example of this is carbon monoxide, in which the partial negative
charge resides on the carbon, as predicted by its negative formal charge
[17, p.
24].
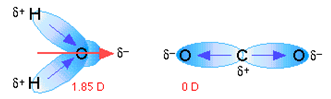
Figure 4.4 Dipole moments of H2O and CO2
Oxidation number is another arbitrary way of characterizing atoms in molecules. In
contrast to formal charge, in which the electrons in a bond are assumed to be
shared equally, oxidation number is
the electric charge an atom would have if the bonding electrons were assigned exclusively to the more
electronegative atom. Oxidation number serves mainly as a tool for
keeping track of electrons in reaction in which they are exhanged between
reactants, and for characterizing the “combining power” of an atom in a molecule
or ion (figure 4.5).
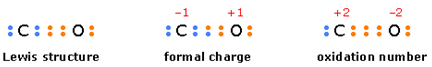
Figure 4.5 Comparison of electron assignments for formal charge and
oxidation number.
Ionic compounds. The shared-electron pair model introduced by G.N. Lewis showed how
chemical bonds could form in the absence of electrostatic attraction
between oppositely-charged ions. As such, it has become the most popular
and generally useful model of bonding in all substances other than
metals. A chemical bond forms when electrons are simultaneously attracted
to two nuclei, thus acting to bind them together in an energeticallystable
arrangement. The covalent bond is formed when two atoms are able to
share a pair of electrons:
A• + B• → A:B
In general, however, different kinds of atoms exert different degrees
of attraction on their electrons, so in most cases the sharing will not be
equal. One can even imagine an extreme case in which the sharing is so unequal
that the resulting “molecule” is simply a pair of ions:
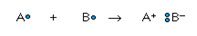
The resulting substance is sometimes said to contain an ionic
bond. Indeed, the properties of a number of compounds can be adequately
explained using the ionic model.
According to the ionic electrostatic model, solids such as NaCl
consist of positive and negative ions arranged in a crystal lattice. Each ion is
attracted to neighboring ions of opposite charge, and is repelled by ions of
like charge; this combination of attractions and repulsions, acting in all
directions, causes the ion to be tightly fixed in its own location in the
crystal lattice.
Since electrostatic forces are nondirectional, the structure of an
ionic solid is determined purely by geometry: two kinds of ions, each with its
own radius, will fall into whatever repeating pattern will achieve the lowest
possible potential energy. Surprisingly, there are only a small number of
possible structures; the very common simple cubic lattice of NaCl is
illustrated here [17, p. 26].
Formation of ionic bond. An ionic bond (also called as electrovalent bond) is a type of
chemical bond that involves a metal ion and a non-metal ion (or
polyatomic ions such as ammonium) through electrostatic attraction. In
short, it is a bond formed by the attraction between two oppositely charged
ions.
The metal donates one or more electrons, forming a positively charged
ion or cation with a stable electron configuration. These electrons then enter
the non-metal, causing it to form a negatively charged ion or anion which also
has a stable electron configuration. The electrostatic attraction between the
oppositely charged ions causes them to come together and form a
bond.
For example, when sodium (Na) and chlorine (Cl) are combined, the
sodium atoms each lose an electron, forming a cation (Na+) and the
chlorine atoms each gain an electron to form an anion (Cl-) (figure
4.6). These ions then are attracted to each other in 1:1 proportion to form
sodium chloride NaCl.
Na + Cl → Na+ + Cl- → NaCl
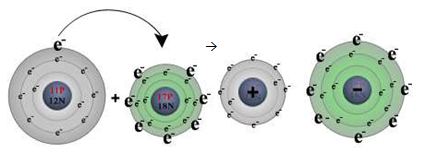
Figure 4.6 Combination of Na and Cl to form Na+ and
Cl-
The figure 4.7 given below shows the variation of potential energy as
a function of distance of separation between sodium or chlorine atoms. An atom
of sodium has one electron extra outside the closed shell and it takes 5.14
electron volts of energy to remove that electron.
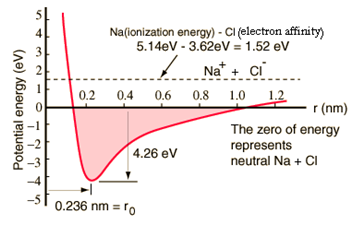
Figure 4.7 P.E. diagram for NaCl molecule
The chlorine atom is short of one electron to fill a shell and it
releases 3.62 electron volts when it acquires that electron (its electron
affinity is 3.62 eV). This means that it takes only 1.52 eV( 5.14 – 3.62 ) of
energy to donate one of the sodium electrons to chlorine when they are far
apart. When the resultant ions are brought close together, their electric
potential becomes more and more negative, reaching – 1.52 eV at about 0.94 nm
separation. This means that if neutral sodium and chlorine atoms found
themselves closer than 0.94 nm, it would be energetically favourable to transfer
electron from Na to Cl and form the ionic bond.
The potential energy curve shows that there is a minimum at 0.236 nm
separation and then a steep rise in potential which represents a repulsive
force. This repulsive force is more than just an electrostatic repulsion between
the electron clouds of the two atoms. The removal of electron from the atom is
endothermic and causes the ions to have a higher energy. There may also be
energy changes associated with breaking of existing bonds or the addition of
more than one electron to form anions. However, the attraction of the ions to
each other lowers their energy.
The energy balance cycle for NaCl is shown
below.
a)
Gaseous sodium atom is formed from solid sodium
metal
Na (s) + 108 kJ mol-1 → Na(g)
b)
Sodium ion is formed from gaseous sodium atom.
Na (g) + 496 kJ mol-1 → Na+ (g) +
e-
c)
Chlorine molecule dissociates into gaseous chlorine
atoms.
½ Cl2 (g) + 121 kJ mol-1 → Cl
(g)
d)
Chloride ion is formed from gaseous chlorine
atom.
Cl (g) + e- → Cl- (g) + 349 kJ
mol-1
e)
Sodium ions and chloride ions interact to form solid sodium
chloride.
Na+ (g) + Cl- (g) → Na+ Cl- (s) + 787 kJ
mol-1
Energy evolved = 349 + 787 = 1136 kJ
-
Energy absorbed = 108 + 496 + 121 = 725 kJ
-----------------------------------------------------------
Energy evolved = 411 kJ mol-1
Ionic bonding will occur only if the overall energy change for the
reaction is favourable – when the bonded atoms have a lower energy than the free
ones. The larger the resulting energy change, the stronger the bond. The low
electronegativity of the metals and high electronegativity of non-metals means
that the energy change of the reaction is most favourable when metals lose
electrons and non-metals gain electrons.
Notice that when sodium loses its one valence electron, it gets
smaller in size, while chlorine grows larger when it gains an additional valence
electron. This is typical of the relative sizes of the ions to atoms. Positive
ions tend to be smaller than the parent atoms while negative ions tend to be
larger than their parent. After the reaction takes place, the charged
Na+ and Cl- ions are held together by electrostatic
forces, thus forming an ionic bond [16, p. 5].
Characteristic Properties of Ionic Compounds. Ionic compounds have following characteristic
properties.
1.
Ionic compounds involve ionic bonds which are formed between metals
and non-metals.
2.
In naming simple ionic compounds, the metal is always first, the
non-metal second ( e.g. sodium chloride )
3.
Ionic compounds dissolve easily in water and other polar
solvents.
4.
In solution and in molten state ionic compounds easily conduct
electricity.
5.
Ionic compounds tend to form crystalline solids with high melting
temperatures.
Pure ionic bonding is not known to exist. All ionic compounds have a
degree of covalent bonding. The larger the difference in electronegativity
between two atoms, the more ionic the bond [16, p. 8].
Metallic bonding. The elements which are placed on the extreme left, the middle and a
few on the right of the periodic table are metals. Alkali metals like
sodium, potassium, alkaline earth metals like magnesium, calcium, transition
metals like iron, cobalt, nickel, copper and others like lead, tin represent the
family of metals. They have low electronegativity. They tend to lose their
valence electrons easily. When we have a macroscopic collection of metal atoms,
the valence electrons are detached from the atoms but not held by any of the
other atoms. In other words, these valence electrons are free from any
particular atom and are held only collectively by the entire assembly of atoms.
When atoms lose their outer-shell electrons they become positive ions. The outer
electrons become a ‘sea’ of mobile electrons surrounding a lattice of positive
ions. The positive ion cores are held more or less at fixed places in an ordered
or crystal lattice. The valence electrons are free to move about under applied
stimulation like electrical field or heat. This is called “electron sea model”
of metals.
The force of attraction which holds the delocalized (or mobile)
electrons and the metallic nuclei together in a metal is called a metallic bond
[16, p. 17].
Characteristic Properties of Metals. Metals show following characteristic physical
properties:
1.
At room temperature, they are solids (except
mercury)
2.
They are opaque to light.
3.
They, generally, have high density.
4.
They show metallic luster.
5.
They are malleable and ductile in their solid
state.
6.
They are good conductors of heat and
electricity.
7.
They have crystal structure in which each atom is surrounded by eight
to twelve near neigh bours [16, p. 18].
Hydrogen bond. This is a different type of bond. It is restricted to only some
molecules containing hydrogen atoms.
The force of attraction between the hydrogen atom attached to an
electronegative atom of one molecule and an electronegative atom of another
molecule is called hydrogen bond.
Usually, the electronegative atom is O, N or F. In a molecule, the O,
N or F atom has a partial negative charge and then the hydrogen atom which has a
very small size has a partial positive charge. This type of bond always involves
hydrogen atom and hence the name hydrogen bond.
In order to form a hydrogen bond, it is necessary that the
electronegative atom should have one or more lone pairs of electrons and a
partial negative charge so that there is a force of attraction termed as
dipole-dipole interaction. The hydrogen atom which has a partial positive charge
tries to find another atom of O, N or F with excess of electrons to share and is
attracted to partial negative charge. This forms the basis of hydrogen
bond.
The hydrogen bond can occur between molecules (intermolecular) like
HF or within different parts of a single molecule (intramolecular) like o-nitro
phenol (figure 4. 8). The hydrogen bond is stronger than van-der-Waals’ bond but
weaker than covalent or ionic bond. The hydrogen bond has the bond energy in the
range 5 to 30 kJ per mole [16, p. 19].
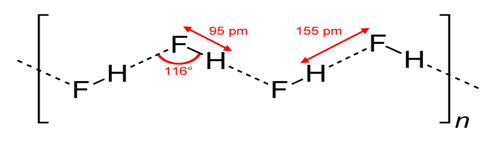
Figure 4.8 Hydrogen bonding in HF
Effects of hydrogen bonding. Hydrogen bonding has effects on the properties of certain
substances.
1.
Hydrogen bonding leads to association of molecules which affects the
physical state of a substance. For example, HF which should be a gas at room
temperature, becomes a liquid due to association of
molecules.
2.
Covalent compounds are normally insoluble in water. But compounds
like ethanol, lower aldehydes, ketones, though covalent, are soluble in water
due to formation of hydrogen bonds with water molecules.
3.
The boiling points of water (100ºC), HF (19.5ºC) and ammonia (- 33ºC)
are exceptionally high as compared to other Group 16 hydrides which have no
hydrogen bonds.
4.
Intramolecular hydrogen bonding is partly responsible for secondary,
tertiary and quaternary structure of proteins and nucleic acids. It also plays
an important role in the structure of polymers [16, p.
20].