The structure of the atom. Mendeleev’s Periodic law and Table of the element
Introduction. Around the 5th
century BC, Greek philosopher Democritus invented the concept of the atom
(from Greek meaning “indivisible”). The atom, eternal, constant,
invisible and indivisible, represented the smallest unit and the building
block of all matter. Democritus suggested that the varieties of matter and
changes in the universe arise from different relations between these most basic
constituents. He illustrated the concept of atom by arguing that every piece of
matter could be cut to an end until the last constituent is reached.
Today the word atom is used to identify the basic component of
molecules that create all matter, but it is known that the atom itself is made
of particles even more fundamental, some of which are elementary. The first
theoretical and experimental models of the structure of matter came as late as
the 19th century, which is the time marked as the beginning of modern
science. At that time a more empirical approach, mainly in chemistry, opened a
new era of scientific investigations.
The work of Democritus remained known through the ages in writings of
other philosophers, mainly Aristotle. Modern Greece has honored Democritus as a
philosopher and the originator of the concept of the atoms through their
currency; the 10-drachma coin, before Greek currency was replaced with the euro,
depicted the face of Democritus on one side, and the schematic of a lithium atom
on the other.
This chapter introduces the structure of atoms and describes atomic
models that show the evidence for the existence of atoms and electrons [13, p.
1].
Atomic models. The cannonball atomic model. All matter on
Earth is made from a combination of 90 naturally occurring different atoms.
Early in the 19th century, scientists began to study the
decomposition of materials and noted that some substances could not be broken
down past a certain point (for instance, once separated into oxygen and
hydrogen, water cannot be broken down any further). These primary substances are
called chemical elements.
By the end of the 19th century it was implicit that matter
can exist in the form of a pure element, chemical compound of two or more
elements or as a mixture of such compounds. Almost 80 elements were known at
that time and a series of experiments provided confirmation that these elements
were composed of atoms.
This led to a discovery of the law of definite proportions:
two elements, when combined to create a pure chemical compound, always combine
in fixed ratios by weight.
For example, if element A combines with element B, the
unification creates a compound AB. Since the weight of A is
constant and the weight of B is constant, the weight ratio of these two
will always be the same. This also implies that two elements will only combine
in the defined proportion; adding an extra quantity of one of the elements will
not produce more of the compound.
Example 3.1: The law of definite
proportion
Carbon (C) forms two compounds when reacting with oxygen (O): carbon
monoxide (CO) and carbon dioxide (CO2).
1g of C + 4/3 g of O → 2 1/3 g of CO; 1 g of C + 8/3 g of O → 3/2 g
of CO2
The two compounds are formed by the combination of a definite number
of carbon atoms with a definite number of oxygen atoms. The ratio of these two
elements is constant for each of the compounds (molecules): C:O = 3:4 for CO and
C:O = 3:8 for CO2.
The first atomic theory with empirical proofs for the law of definite
proportion was developed in 1803 by the English chemist John Dalton (1766–1844).
Dalton conducted a number of experiments on gases and liquids and concluded
that, in chemical reactions, the amount of the elements combining to form a
compound is always in the same proportion.
He showed that matter is composed of atoms and that atoms have their
own distinct weight. Although some explanations in Dalton’s original atomic
theory are incorrect, his concept that chemical reactions can be explained by
the union and separation of atoms (which have characteristic properties)
represents the foundations of modern atomic physics. In his two-volume book,
New System of Chemical Philosophy, Dalton suggested a way to
explain the new experimental chemistry. His atomic model described how all
elements were composed of indivisible particles which he called atoms (he
depicted atoms like cannonballs, figure 3.1) and that all atoms of a given
element were exactly alike.
This explained the law of definite proportions. Dalton further
explained that different elements have different atoms and that compounds were
formed by joining the atoms of two or more elements.
In 1811, Amadeo Avogadro, conte di Quaregna e Ceretto (1776–1856),
postulated that equal volumes of gases at the same temperature and pressure
contain the same number of molecules. Sadly, his hypothesis was not proven until
2 years after his death at the first international conference on chemistry held
in Germany in 1860 where his colleague, Stanislao Cannizzaro, showed the system
of atomic and molecular weights based on Avogadro’s
postulates.
Example 3.2: Avogadro’s
law
As shown in Example 3.1, the ratio of carbon and oxygen in forming
CO2 is 3:8. Here is the explanation of this ratio: since a single
atom of carbon has the same mass as 12 hydrogen atoms, and two oxygen atoms have
the same mass as 32 hydrogen atoms, the ratio of the masses is 12:32 = 3:8. This
shows that the description of the reaction is independent of the units used
since it is the ratio of the masses that determines the outcome of a chemical
reaction.
Thus, whenever you see wood burning in a fire, you should know that
for every atom of carbon from the wood, two oxygen atoms from the air are
combined to form CO2; the ratio of masses is always
12:32.
It follows that there must be as many carbon atoms in 12 g of carbon
as there are oxygen atoms in 16 g of oxygen. This measure of the number of atoms
is called a mole. The mole is used as a convenient measure of an amount
of matter, similarly as “a dozen” is a convenient measure of 12 objects of any
kind. Thus, the number of atoms (or molecules) in a mole of any substance is the
same. This number is called Avogadro’s number (NA) and its value was
accurately measured in the 20th century as 6.02 × 1023 atoms or
molecules per mole.
For example, the number of moles of hydrogen atoms in a sample that
contains 3.02 × 1021 hydrogen atoms is
Moles of H atoms = 3,02×1021 atoms
H/6,02×1023atoms/mole = 5.01×10-3 moles H [13, p. 2]
Figure 3.1 Cannonball atomic model (John Dalton,
1803)
The Plum Pudding Atomic Model. Shortly before the end of
the 19th century, a series of new experiments and discoveries opened
the way for new developments in atomic and subatomic (nuclear) physics. In
November 1895, Wilhelm Roentgen (1845– 1923) discovered a new type of radiation
called X-rays, and their ability to penetrate highly dense materials.
Soon after the discovery of X-rays, Henri Becquerel (1852–1908) showed that
certain materials emit similar rays independent of any external force. Such
emission of radiation became known as
radioactivity.
During this same time period, scientists were extensively studying a
phenomenon called cathode rays. Cathode rays are produced between two
plates (a cathode and an anode) in a glass tube filled with the very low-density
gas when an electrical current is passed from the cathode to the high-voltage
anode. Because the glowing discharge forms around the cathode and then extends
toward the anode, it was thought that the rays were coming out of the cathode.
The real nature of cathode rays was not understood until 1897 when Sir Joseph
John Thomson (1856–1940) performed experiments that led to the discovery of the
first subatomic particle, the electron. The most important aspect of his
discovery is that the cathode rays are the stream of particles.
Here is the explanation of his postulate: from the experiment he observed that
cathode rays were always deflected by an electric field from the negatively
charged plate inside the cathode ray tube, which led him to conclude that the
rays carried a negative electric charge. He was able to determine
the speed of these particles and obtain a value that was a fraction of the speed
of light (one tenth the speed of light, or roughly 30,000 km/s or 18,000 mi/s).
He postulated that anything that carries a charge must be of material origin and
composed of particles. In his experiment, Thomson was able to measure the
charge-to-mass ratio, e/m, of the cathode rays; a property that
was found to be constant regardless of the materials used. This ratio was also
known for atoms from electrochemical analysis, and by comparing the values
obtained for the electrons he could conclude that the electron was a very small
particle, approximately 1,000 times smaller than the smallest atom (hydrogen).
The electron was the first subatomic particle identified and the fastest small
piece of matter known at that time.
In 1904, Thomson developed an atomic model to explain how the
negative charge (electrons) and positive charge (speculated to exist since it
was known that the atoms were electrically neutral) were distributed in an atom.
He concluded that the atom was a sphere of positively charged material with
electrons spread equally throughout like raisins in a plum pudding. Hence, his
model is referred to as the plum pudding model, or raisin bun atom
as depicted in figure 3.2. This model could explain
·
The neutrality of atoms
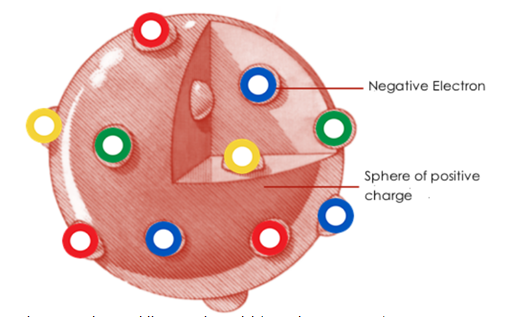
Figure 3.2 Plum pudding atomic model (J. J. Thomson,
1904)
·
The origin of electrons
·
The origin of the chemical properties of elements. However, his model
could not answer questions regarding
·
Spectral lines (according to this model, radiation emitted should be
monochromatic; however, experiments with hydrogen shows a series of lines
falling into different parts of the electromagnetic
spectrum)
·
Radioactivity (nature of emitted rays and their origin in the atom).
Scattering of charged particles by atoms
Thomson won the Nobel Prize in 1906 for his discovery of the
electron. He worked in the famous Cavendish Laboratory in Cambridge and was one
of the most influential scientists of his time. Seven of his students and
collaborators won Nobel Prizes, among them his son who, interestingly, won the
Nobel Prize for proving the electron is a wave [13, p. 4].
The Planetary Atomic Model. Disproof of Thomson’s Plum
Pudding Atomic Model. Thomson’s atomic model described the atom as a relatively
large, positively charged, amorphous mass of a spherical shape with negatively
charged electrons homogeneously distributed throughout the volume of a sphere,
the sizes of which were known to be on the order of an Ångström (1 Å =
10-8 cm = 10-10 m). In 1911 Geiger and Marsden carried out
a number of experiments under the direction of Ernest Rutherford (1871–1937) who
received the Nobel Prize in chemistry in 1908 for investigating and classifying
radioactivity. He actually did his most important work after he received the
Nobel Prize and the 1911 experiment unlocked the hidden nature of the atom
structure.
Rutherford placed a naturally radioactive source (such as radium)
inside a lead block as shown in figure 3.3. The source produced α
particles which were collimated into a beam and directed toward a
thin gold foil. Rutherford hypothesized that if Thomson’s model was correct then
the stream of α particles would pass straight through the foil with only a few being
slightly deflected as illustrated in figure 3.4. The “pass through” the atom
volume was expected because the Thomson model postulated a rather uniform
distribution of positive and negative charges throughout the atom. The
deflections would occur when the positively charged α
particles came very close to the individual electrons or the regions
of positive charges. As expected, most of the α
particles went through the gold foil with almost no deflection.
However, some of them rebounded almost directly backward – a phenomenon that was not expected (figure 3.5).
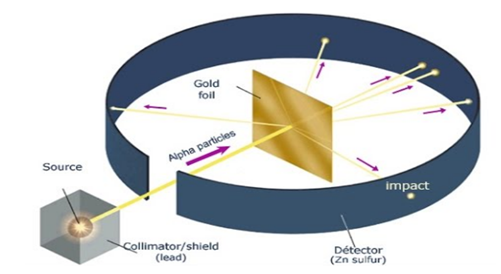
Figure 3.3 Schematics of Rutherford’s
experiment (1911)
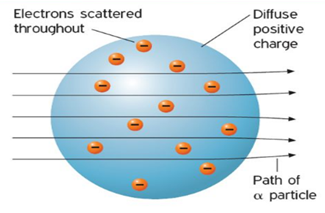
Figure 3.4 Expected scattering of
α
particles in Rutherford’s experiment
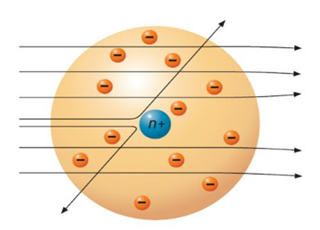
Figure 3.5 Actual scattering of
α
particles in Rutherford’s experiment
Rutherford explained that most of the α
particles pass through the gold foil with little or no divergence not
because the atom is a uniform mixture of the positive and negative charges but
because the atom is largely empty space and there is nothing to interact with
the α
particles. He explained the large scattering angle by suggesting that
some of the particles occasionally collide with, or come very close to, the
“massive” positively charged nucleus that is located at the center of an
atom. It was known at the time that the gold nucleus had a positive charge of 79
units and a mass of about 197 units while the α
particle had a positive charge of 2 units and a mass of 4 units. The
repulsive force between the α
particle and the gold nucleus is proportional to the product of their
charges and inversely proportional to the square of the distance between them.
In a direct collision, the massive gold nucleus would hardly be moved by the
α
particle. The diameter of the nucleus was shown to be ~1/105 the size
of the atom itself, or ~10−13 m. Clearly these ideas defined an atom very different from the
Thomson’s model.
Ernest Solvay (1838–1922), a Belgian industrial chemist, who made a
fortune from the development of a new process to make washing soda (1863), was
known for his generous financial support to science, especially physics research. Among the projects he financially supported, was a
series of international conferences, known as the Solvay conferences. The
First Solvay Conference on Physics was held in Brussels in 1911
and it was attended by the most famous scientists of the time. Rutherford was
one of them; he presented the discovery of the atomic nucleus and explained the
structure of the atom. According to his explanation, the electrons revolve
around the nucleus at relatively large distances. Since each electron carries
one elementary charge of negative electricity, the number of electrons must
equal the number of elementary charges of positive electricity carried by the
nucleus for the atom to be electrically neutral. The visual model is similar to
the solar planetary system and is illustrated in figure 3.6 [3, p.7].
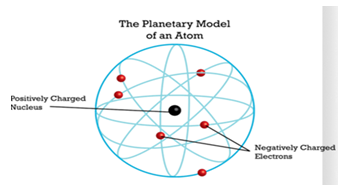
Figure 3.6 Planetary atomic model (Rutherford, 1911)
The Smallness of the Atom. Rutherford’s gold foil
experiment was the first indication and proof that the space occupied by an atom
is huge compared to that occupied by its nucleus. In fact, the electrons
orbiting the nucleus can be compared to a few flies in a cathedral. As a
qualitative reference, a human is about two million times “taller” than the
average Escherichia coli bacterium; Mount Everest is about 5,000 times
taller than the average man; and a man is about ten billion times “taller” than
the oxygen atom. If the atom were scaled up to a size of a golf ball, on that
same scale a man would stretch from Earth to the Moon. Atoms are so small that
direct visualization of their structure is impossible. Today’s best optical or
electron microscopes cannot reveal the interior of an
atom.
The picture shown in figure 3.7 was taken with a scanning
transmission electron microscope and shows a direct observation of cubes of
magnesium oxide molecules, but details of the atoms cannot be
seen.
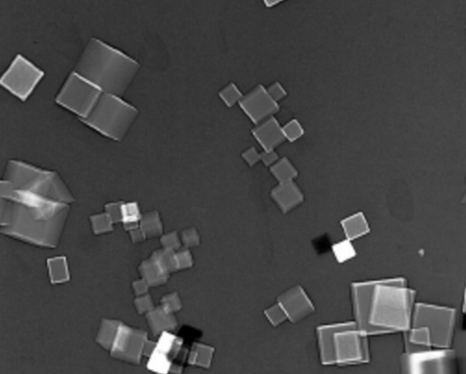
Figure 3.7 Magnesium oxide molecules as seen with scanning
transmission electronic microscope produced at the Institute of Standards and
Technology in the USA (Courtesy National Institute of Standards and
Technology)
The image shown in figure 3.8 represents an 8 nm square structure
with cobalt atoms arranged on a copper surface. Such arrangements of atoms are
used to investigate the physics of ultra-tiny objects. The shown structure was
observed with a scanning tunneling microscope at a temperature of 2.3 K (about
−455º F): the larger peaks (upper left and lower right) are pairs of
cobalt atoms, while the two smaller peaks are single cobalt atoms. The swirls on
the copper surface illustrate how the cobalt and copper electrons interact with
each other [13, p. 20].
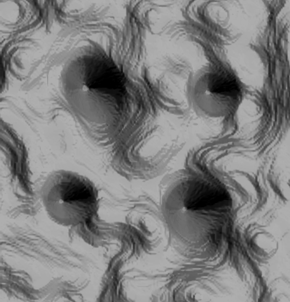
Figure 3.8 Nanoscale structure of cobalt and copper atoms produced at the
Institute of Standards and Technology in the USA (Courtesy of J. Stroscio, R.
Celotta, A. Fein, E. Hudson, and S. Blankenship, 2002)
The Quantum Atomic Model. Quantum Leap. In 1913, Niels Bohr (1885–1962)
developed the atomic model that resolved Rutherford’s atomic stability
questions. His model was based on the work of Planck (energy quantization),
Einstein (photon nature of light) and Rutherford (nucleus at the center of the
atom).
In 1900, Max Planck (1858–1947) resolved the long-standing problem of
black body radiation by showing that atoms emit light in bundles of radiation
(called photons by Einstein in 1905 in his theory of the photoelectric
effect). This led to formulation of Planck’s radiation law: a light is
emitted as well as absorbed in discrete quanta of energy. The magnitude
of these discrete energy quanta is proportional to the light’s frequency
(f, which represents the color of light) (1):
E = hf = hc/𝜆 (1)
where h is Planck’s constant (h = 6.63 × 10−34 J s), c is the speed of light and λ
is the wavelength of the emitted or absorbed light. Bohr applied this
quantum theory of light to the structure of the electrons by restricting them to
exist only along certain orbits (called the allowed orbits) and not
allowing them to appear at arbitrary locations inside the atom. The
angular momentum of the electrons is quantized and thus prohibits random
trajectories around the nucleus. Consequently the electrons cannot emit
or absorb electromagnetic radiation in arbitrary amounts since an
arbitrary amount would lead to an energy that would force the electron to
move to an orbit that does not exist. Electrons are thus allowed to move
from one orbit to another. However, the electrons never actually cross
the space between the orbits. They simply appear or disappear within the
allowed states; a phenomenon referred to as a quantum leap or
quantum jump.
For his theory of atoms that introduced the new discipline of quantum
mechanics in physics, Bohr received a Noble Prize in 1922. He was also a founder
of the Copenhagen school of quantum mechanics. One of his students once noticed
a horseshoe nailed above his cabin door and asked him: “Surely, Professor Bohr,
you don’t believe in all that silliness about the horseshoe bringing good luck?”
With a gentle smile Bohr replied, “No, no, of course not, but I understand that
it works whether you believe it or not” [13, p. 21].
Atoms of Higher Z. Quantum Numbers. The light
spectra of atoms with more than one electron are much more complex than that of
the hydrogen atom (many more lines). The calculations of the spectra for these
atoms with the Bohr atomic model are complicated by the screening effect of the
other electrons. Examination of the hydrogen spectral lines with high-resolution
spectroscopes shows these lines to have very fine structures, and the observed
spectral lines are each actually made up of several lines that are very close
together. This observation implied the existence of sublevels of energy within
the principal energy level, which makes Bohr’s theory inadequate even for the
hydrogen atomic spectrum.
Bohr recognized that the electrons are most likely organized into
orbital groups in which some are close and tightly bound to the nucleus, and
others less tightly bound at larger orbits. He proposed a classification scheme
that groups the electrons of multi-electron atoms into “shells” and each shell
corresponds to a so-called quantum number n. These shells are given names
that correspond to the values of the principal quantum
numbers:
·
n = 1 (K shell) can hold no more than 2
electrons
·
n = 2 (L shell) can hold no more than 8
electrons
·
n = 3 (M shell) can hold no more than 18 electrons,
etc.
Moseley’s work contributed to the understanding that the electrons in
an atom existed in groups visualized as electron shells, and according to
quantum mechanics, the electrons are distributed around the nucleus in
probability regions also called the atomic orbitals.
In order to completely describe an atom in three dimensions,
Schrödinger introduced three quantum numbers in addition to the principal
quantum number, n. There are thus a total of four quantum numbers that
specify the behavior of electrons in an atom, namely
·
principal quantum number, n = 1, 2, 3,
…
·
azimuthal quantum number, l = 0 to n – 1
·
magnetic quantum number, m = −l to 0 to +l
·
spin quantum number, s = −1/2 or +1/2.
The principal quantum number describes the shells in which the
electrons orbit. The maximum number of electrons in a shell n is
2n2.
The sub-energy
levels (s, p, d, etc.) are the reason for the very fine
structure of the spectral lines and result from the electron’s rotation around
the nucleus along elliptical (not circular) orbits.
The azimuthal quantum number describes the actual shape of the
orbits.
For example, l = 0 refers to a spherically shaped orbit, l
= 1 refers to two obloid spheroids tangent to one another and l = 2
indicates a shape that is quadra-lobed (similar to a four leaf clover). For a
given principal quantum number, n, the maximum number of electrons in an
l = 0 orbital is 2, for an l = 1 orbital it is 6 and an l =
2 orbital can accommodate a maximum of 10 electrons.
The magnetic quantum number is also referred to as the
orbital quantum number and it physically represents the orbital’s
direction in space. For example when l = 0, m can only be
zero. This single value for the magnetic quantum number suggests a single
spatial direction for the orbital. A sphere is uni-directional and it
extends equally in all directions, thus the reason for a single m
value. If l = 1 then m can be assigned the values −1, 0 or +1. The three values for m suggest that the
double-lobed orbital has three distinctly different directions in
three-dimensional space into which it can extend. In the absence of any
perturbing force (such could be an external magnetic field) the orbitals with
the same n and l are equal in energy and are called
degenerate.
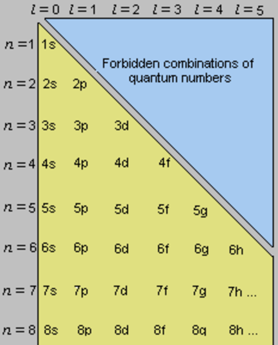
Figure 3.9 Allowed combinations of quantum numbers
In the presence of a perturbing force caused by the magnetic field
the orbitals would differ in energy, and thus this quantum number is called the
magnetic quantum number.
The spin quantum number describes the spin of the electrons.
The electrons spin around an imaginary axis (as Earth spins about the imaginary
axis connecting the north and south poles) in a clockwise or counterclockwise
direction; for this reason there are two values, −1/2 or +1/2. The allowed combination of quantum numbers is given in
figure 3.9 [13, p. 39].
The Wave Nature of Light. If you drop a stone into one end of a
quiet pond, the impact of the stone with the water starts an up-and-down motion
of the water surface. This up-and-down motion travels outward from where the
stone hit; it is a familiar example of a wave. A wave is a continuously repeating change or oscillation in matter or in a
physical field. Light is also a wave. It consists of oscillations in electric
and magnetic fields that can travel through space. Visible light, x rays, and
radio waves are all forms of electromagnetic radiation.
You characterize a wave by its wavelength and frequency.
The wavelength, denoted by the Greek letter λ (lambda), is the distance between any two adjacent identical points of a wave. Thus, the wavelength is the distance between two adjacent peaks or
troughs of a wave. figure 3.3 shows a cross section of a water wave at a given
moment, with the wavelength (λ) identified. Radio waves have wavelengths from approximately 100 mm
to several hundred meters. Visible light has much shorter wavelengths, about
10-6m. Wavelengths of visible light are often given in nanometers (1
nm = 10-9 m). For example, light of wavelength 5.55
∙ 10-7 m, the greenish yellow light to which
the human eye is most sensitive, equals 555 nm.
The frequency of a wave is the number of wavelengths of that wave that pass a fixed point in one
unit of time (usually one second). For example, imagine you are anchored in a small boat on a pond when a stone is dropped into the
water. Waves travel outward from this point and move past your boat. The number of
wavelengths that pass you in one second is the frequency of that wave. Frequency
is denoted by the Greek letter v (nu,
pronounced “new”). The unit of frequency is /s, or s-1,
also called the hertz (Hz).
The wavelength and frequency of a wave are related to each other.
figure 3.4 shows two waves, each traveling from left to right at the same speed;
that is, each wave moves the same total length in 1s. The top wave, however, has
a wavelength twice that of the bottom wave. In 1s, two complete wavelengths of
the top wave move left to right from the origin. It has a frequency of 2/s, or 2
Hz. In the same time, four complete wavelengths of the bottom wave move left to
right from the origin. It has a frequency of 4/s, or 4 Hz. Note that for two
waves traveling with a given speed, wavelength and frequency are inversely
related: the greater the wavelength, the lower the frequency, and vice versa. In
general, with a wave of frequency v
and wavelength λ, there are v
wavelengths, each of length λ, that pass a fixed point every second. The
product vλ is the total length of the wave
that has passed the point in 1s. This length of wave per second is the speed of
the wave. For light of speed c (2),
c = vλ (2)
The speed of light waves in a vacuum is a constant and is independent
of wavelength or frequency. This speed is 3.00 ∙ 108 m/s, which is
the value for c that we use in the following examples. The range of frequencies or wavelengths of electromagnetic radiation
is called the electromagnetic spectrum, shown in figure 3.5. Visible light extends from the violet end of the
spectrum, which has a wavelength of about 400 nm, to the red end, with a
wavelength of less than 800 nm. Beyond these extremes, electromagnetic radiation
is not visible to the human eye. Infrared radiation has wavelengths greater than
800 nm (greater than the wavelength of red light), and ultraviolet radiation has
wavelengths less than 400 nm (less than the wavelength of violet light) [15, p.
265].
Quantum Effects and Photons. Isaac Newton, who studied the
properties of light in the seventeenth century, believed that light consisted of
a beam of particles. In 1801, however, British physicist Thomas Young showed
that light, like waves, could be diffracted. Diffraction is a property of waves in which the waves spread out when they
encounter an obstruction or small hole about the size of the wavelength. You can
observe diffraction by viewing a light source through a hole—for example, a
streetlight through a mesh curtain. The image of the streetlight is blurred by
diffraction.
By the early part of the twentieth century, the wave theory of light
appeared to be well entrenched. But in 1905 the German physicist Albert Einstein
(1879–1955; emigrated to the United States in 1933) discovered that he could
explain a phenomenon known as the photoelectric effect by postulating that light had
both wave and particle properties. Einstein based this idea on the work of the
German physicist Max Planck (1858–1947) [15, p. 268].
Planck’s Quantization of Energy. In 1900 Max Planck found
a theoretical formula that exactly describes the intensity of light of various
frequencies emitted by a hot solid at different temperatures. Earlier, others
had shown experimentally that the light of maximum intensity from a hot solid
varies in a definite way with temperature. A solid glows red at 750_C, then white as the temperature increases to 1200ºC.
At the lower temperature, chiefly red light is emitted. As the temperature
increases, more yellow and blue light become mixed with the red, giving white
light.
According to Planck, the atoms of the solid oscillate, or
vibrate, with a definite frequency v,
depending on the solid. But in order to reproduce the results of experiments on
glowing solids, he found it necessary to accept a strange idea. An atom could
have only certain energies of vibration, E, those allowed by the formula
(3)
E = nhv, n =1, 2, 3, . . . (3)
where h is a constant, now called Planck’s constant, a
physical constant relating energy and frequency, having the value 6.63 ∙
10-34J∙s. The value of n must be 1 or 2 or some
other whole number. Thus, the only energies a vibrating atom can have are
h_, 2h_, 3h_, and so forth.
The numbers symbolized by n are called quantum numbers.
The vibrational energies of the atoms are said to be quantized; that is,
the possible energies are limited to certain values.
The quantization of energy seems contradicted by everyday experience.
Consider the potential energy of an object, such as a tennis ball. Its potential
energy depends on its height above the surface of the earth: the greater the
upward height, the greater the potential energy. (Recall the discussion of
potential energy in Section 6.1.) We have no problem in placing the tennis ball
at any height, so it can have any energy. Imagine, however, that you could only
place the tennis ball on the steps of a stairway. In that case, you could only
put the tennis ball on one of the steps, so the potential energy of the tennis
ball could have only certain values; its energy would be quantized. Of course,
this restriction of the tennis ball is artificial; in fact, a tennis ball can
have a range of energies, not just particular values. As we will see, quantum
effects depend on the mass of the object: the smaller the mass, the more likely
you will see quantum effects. Atoms, and particularly electrons, have small
enough masses to exhibit quantization of energy; tennis balls do not [15, p.
268].
Photoelectric Effect. Planck himself was uneasy with the
quantization assumption and tried unsuccessfully to eliminate it from his
theory. Albert Einstein, on the other hand, boldly extended Planck’s work to
include the structure of light itself. Einstein reasoned that if a vibrating
atom changed energy, say from 3h to 2h, it would decrease in energy by h, and this energy would be emitted as a bit (or
quantum) of light energy. He therefore postulated that light consists of quanta
(now called photons), or particles of electromagnetic energy, with energy E proportional to the observed frequency of the
light (4):
E = hv (4)
In 1905 Einstein used this photon concept to explain the
photoelectric effect.
The photoelectric effect is the ejection of electrons from the surface of a metal or from another
material when light shines on it (see figure 3.6). Electrons are ejected, however, only when the frequency of light exceeds a certain
threshold value characteristic of the particular metal. For example, although violet light will
cause potassium metal to eject electrons, no amount of red light (which has a lower
frequency) has any effect.
To explain this dependence of the photoelectric effect on the
frequency, Einstein assumed that an electron is ejected from a metal when it is
struck by a single photon. Therefore, this photon must have at least enough
energy to remove the electron from the attractive forces of the metal. No matter
how many photons strike the metal, if no single one has sufficient energy, an
electron cannot be ejected. A photon of red light has insufficient energy to
remove an electron from potassium. But a photon corresponding to the threshold
frequency has just enough energy, and at higher frequencies it has more than
enough energy. When the photon hits the metal, its energy h is taken up by the electron. The photon ceases to exist as a
particle; it is said to be absorbed.
The wave and particle pictures of light should be regarded as
complementary views of the same physical entity. This is called the
wave–particle duality of light. The equation E = hv displays this duality; E is the energy of a light particle or photon, and v is the frequency of the associated wave. Neither the wave nor the
particle view alone is a complete description of light [15, p.
26].
Sizes of Atoms and Ions. In earlier chapters we discovered
the importance of atomic masses in matters relating to
stoichiometry. To understand certain physical and chemical properties,
we need to know something about atomic sizes. In this section we
describe atomic radius, the first of a group of atomic properties
that we will examine in this chapter
Atomic Radius. The size of an atom, expressed as the
atomic radius, represents the distance between the nucleus and the valence, or
outermost, electrons. The boundary between the nucleus and the electrons is not
easy to determine and the atomic radius is therefore approximated. For example,
the distance between the two chlorine atoms of Cl2 is known to be
nearly 2Å. In order to obtain the atomic radius, the distance between the two
nuclei is assumed to be the sum of the radii of two chlorine atoms. Therefore
the atomic radius of chlorine is ~1Å (or 100 pm, see figure
3.10).
The atomic radius changes across the periodic table of elements and
is dependent on the atomic number and the electron distribution. Since electrons
repel each other due to like charges, the overall size of the atom increases
with an increase in the number of electrons in each of the groups (see figure
3.10). For example, the radius of a hydrogen atom is smaller than the radius of
the lithium atom. The outer electron of lithium is in the n = 2 level, so
its radius must be larger than the radius of hydrogen which has its outermost
electron in the n = 1 level. However, in spite of the increase in the
number of electrons, the atomic radius decreases when going from left to right
across the periodic table. This is a result of an increase in the number of
protons for these elements, which all have their valence electrons in the same
quantum energy level. Since the electrons are attracted to the protons, the
increased charge of the nucleus (more protons) binds the electrons more tightly
and brings them closer to the nucleus, causing the overall atomic radius to
decrease. For example, the first two elements in the second period of the
periodic table are lithium and beryllium.
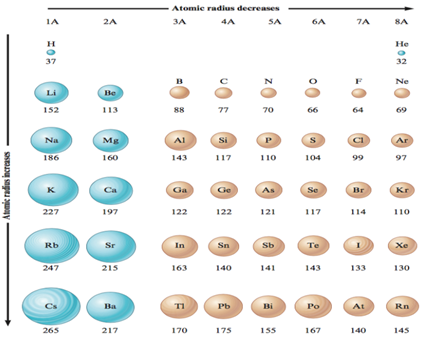
Figure 3.10 Trends of atomic radii (listed in picometers) in the
periodic table
The radius of a beryllium atom is 113 pm, which is smaller than that
of lithium (152 pm). In beryllium, Z = 4, the fourth electron joins the
third in the 2s level, assuming their spins are anti-parallel. The charge
is thus larger and this causes the electrons to be bound more tightly to the
nucleus; as a result the beryllium radius is less than the lithium radius. The
effect of the increased charge should, however, be seen in the context of the
quantum energy levels. For example, cesium has a large number of protons but it
is one of the largest atoms. The valence electrons are furthest from the nucleus
and the inner electrons shield them from the positive charge of the nucleus;
thus the valence electrons experience a reduced effective nuclear charge and not
the total charge of the nucleus. The effect of the increase in the nuclear
charge thus only plays a role in the periods from left to right, e.g., from
sodium to argon in the third period, since the additional valence electrons (in
the same quantum energy level) are exposed to a greater effective nuclear
charge along the period [13, p. 49].
Ionic Radius. When a metal atom loses one or more
electrons to formion, the positive nuclear charge exceeds the negative charge of
the electrons in the resulting cation. The nucleus draws the electrons in
closer, and, as a consequence, the following holds true.
Cations are smaller than the atoms from which they are formed.
For isoelectronic cations, the more positive the ionic charge, the
smaller the ionic radius.
Anions are larger than the atoms from which they are formed. For
isoelectronic anions, the more negative the charge, the larger the ionic radius
[3, p.372].
Ionization Energy. In discussing metals, we talked about
metal atoms losing electrons and thereby altering their electron configurations.
But atoms do not eject electrons spontaneously. Electrons are attracted to the
positive charge on the nucleus of an atom, and energy is needed to overcome that
attraction. The more easily its electrons are lost, the more metallic an atom is
considered to be. The ionization energy, I, is the quantity of energy a
gaseous atom must absorb to be able to expel an electron. The electron
that is lost is the one that is most loosely held.
Ionization energies are usually measured through experiments based on
the photoelectric effect in which gaseous atoms at low pressures are bombarded
with photons of sufficient energy to eject an electron from the atom. Here are
two typical values.
Mg(g) → Mg+(g) + e-
I1 = 738 kJ/mol
Mg+(g) →
Mg2+(g) + e- I2
= 1451 kJ/mol
The symbol l1 stands for the first ionization energy the
energy required to strip one electron from a neutral gaseous atom. I2
is the second ionization
energy - the energy to strip an electron from a gaseous ion with a charge of
Further ionization energies are I3, I4, and so on. Each succeeding ionization
energy is invariably larger than the preceding one. In the case of magnesium,
for example, in the second ionization, the electron, once freed, has to move
away from an ion with a charge of +2 (Mg2+). More energy must be
invested than for a freed electron to move away from an ion with a charge of
+1(Mg+). This is a direct consequence of Coulomb s law, which states,
in part, that the force of attraction between oppositely charged particles is
directly proportional to the magnitudes of the charges.
Ionization energies decrease as atomic radii
increase.
This observation that ionization energies decrease as atomic radii
increase reflects the effect of n and Zeff2 on the
ionization energy (I) (5).
I= RH × Z2eff/n2
(5)
so that across a period, as Zeff increases and the
valence-shell principal quantum number n remains constant, the ionization
energy should increase. And down a group, as n increases and
Zeff increases only slightly, the ionization energy should decrease.
Thus, atoms lose electrons more easily (become more metallic) as we move from
top to bottom in a group of the periodic table [3, p.
374].
Magnetic Properties. An important property related to the
electron configurations of atoms and ions is their behavior in a
magnetic field. A spinning electron is an electric charge in
motion. It induces a magnetic field (recall the discussion on page
334). In a diamagnetic atom or ion, all electrons are paired and
the individual magnetic effects cancel out. A diamagnetic species
is weakly repelled by a magnetic field. A paramagnetic atom or ion
has unpaired electrons, and the individual magnetic effects do not
cancel out. The unpaired electrons possess a magnetic moment that
causes the atom or ion to be attracted to an external magnetic
field. The more unpaired electrons present, the stronger is this
attraction.
Manganese has a paramagnetism corresponding to five
unpaired electrons, which is consistent with the electron
configuration
Mn: [Ar]
|
↑ |
↑ |
↑ |
↑ |
↑ |
|
↑↓ |
3d
4s
When a manganese atom loses two electrons, it becomes the ion
Mn2+ which is paramagnetic, and the strength of its paramagnetism
corresponds to five unpaired electrons.
Mn2+: [Ar]
|
↑ |
↑ |
↑ |
↑ |
↑ |
|
|
3d
4s
When a third electron is lost to produce Mn3+, the ion has
a paramagnetism corresponding to four unpaired electrons. The third electron
lost is one of the unpaired 3d electrons [3, p. 379].
Mn3+: [Ar]
|
↑ |
↑ |
↑ |
↑ |
|
|
|
3d
4s
Elements and Periodicity. The elements are found in
various states of matter and define the independent constituents of atoms, ions,
simple substances, and compounds. Isotopes with the same atomic number belong to
the same element. When the elements are classified into groups according to the
similarity of their properties as atoms or compounds, the periodic table of the
elements emerges. Chemistry has accomplished rapid progress in understanding the
properties of all of the elements. The periodic table has played a major role in
the discovery of new substances, as well as in the classification and
arrangement of our accumulated chemical knowledge. The periodic table of the
elements is the greatest table in chemistry and holds the key to the development
of material science. Inorganic compounds are classified into molecular compounds
and solid-state compounds according to the types of atomic arrangements [14,
p.1].
The origin of elements and their distribution. All
substances in the universe are made of elements. According to the current
generally accepted theory, hydrogen and helium were generated first immediately
after the Big Bang, some 15 billion years ago. Subsequently, after the elements
below iron (Z = 26) were formed by nuclear fusion in the incipient stars,
heavier elements were produced by the complicated nuclear reactions that
accompanied stellar generation and decay.
In the universe, hydrogen (77 %) and helium (21 %) are overwhelmingly
abundant and the other elements combined amount to only 2%. Elements are
arranged below in the order of their abundance,
11H˃42He˃168O˃126C˃2014Ne˃2814Si˃2713Al˃2412Mg˃5626Fe
The atomic number of a given element is written as a left subscript
and its mass number as a left superscript [14, p.6].
Discovery of elements. The long-held belief that all
materials consist of atoms was only proven recently, although elements, such as
carbon, sulfur, iron, copper, silver, gold, mercury, lead, and tin, had long
been regarded as being atom-like. Precisely what constituted an element was
recognized as modern chemistry grew through the time of alchemy, and about 25
elements were known by the end of the 18th century. About 60 elements had been
identified by the middle of the 19th century, and the periodicity of their
properties had been observed.
The element technetium (Z = 43), which was missing in the periodic
table, was synthesized by nuclear reaction of Mo in 1937, and the last
undiscovered element promethium (Z = 61) was found in the fission products of
uranium in 1947. Neptunium (Z = 93), an element of atomic number larger than
uranium (Z = 92), was synthesized for the first time in 1940. There are 103
named elements. Although the existence of elements Z = 104-111 has been
confirmed, they are not significant in inorganic chemistry as they are produced
in insufficient quantity.
All trans-uranium elements are radioactive, and among the elements
with atomic number smaller than Z = 92, technetium, prometium, and the elements
after polonium are also radioactive. The half-lives (refer to Section 7.2) of
polonium, astatine, radon, actinium, and protoactinium are very short.
Considerable amounts of technetium 99Tc are obtained from fission products.
Since it is a radioactive element, handling 99Tc is problematic, as it is for
other radioactive isotopes, and their general chemistry is much less developed
than those of manganese and rhenium in the same group. Atoms are equivalent to
alphabets in languages, and all materials are made of a combination of elements,
just as sentences are written using only 26 letters [14,
p.7].
Electronic structure of elements. Wave functions of
electrons in an atom are called atomic orbitals. An atomic orbital is expressed
using three quantum numbers; the principal quantum number, n; the azimuthal quantum number, l; and the magnetic quantum number, ml. For a principal quantum
number n, there are n azimuthal quantum numbers l ranging from 0 to n-1, and each corresponds to the
following orbitals.
l : 0, 1, 2, 3, 4, …
s, p, d, f,
g, …
An atomic orbital is expressed by the combination of n and l. For example, n is 3 and l is 2 for a 3d orbital. There are 2l+1 ml values, namely l, l-1, l-2, ..., -l. Consequently, there are one s orbital, three p orbitals, five d orbitals and seven f orbitals. The three aforementioned
quantum numbers are used to express the distribution of the electrons in
hydrogen-type atom, and another quantum number ms (1/2, -1/2)
which describes the direction of an electron spin is necessary to completely
describe an electronic state.
Therefore, an electronic state is defined by four quantum numbers (n, l, ml,
ms).
The wave function ψ which determines the orbital shape can be expressed as the product
of a radial wave function R and an angular wave function Y as follows
(6).
ψn,l,ml = Rn,l(r)Yl,ml(θ,φ) (6)
R is a function of distance from the nucleus, and Y expresses the
angular component of the orbital. Orbital shapes are shown in figure 3.11. Since
the probability of the electron’s existence is proportional to the square of the
wave function, an electron density map resembles that of a wave function. The
following conditions must be satisfied when each orbital is filled with
electrons.
Pauli principle: The number of electrons that are allowed
to occupy an orbital must be limited to one or two, and, for the latter case,
their spins must be anti-parallel (different direction).
Hund's rule: When there are equal-energy orbitals,
electrons occupy separate orbitals are their spins are parallel (same
direction).
The order of orbital energy of a neutral atom
is
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p
…
and the electron configuration is determined as electrons occupy
orbitals in this order according to the Pauli principle and Hund's rule. An s
orbital with one ml
can accommodate 2 electrons, a p orbital with three ml 6 electrons, and
a d orbital with five ml 10
electrons.
C:
1s22s22p2 or
[He] 2s22p2
Fe: 1s22s22p63s23p63d64s2 or
[Ar] 3d64s2
Au: 1s2 2s2 2p6
3s2 3p6 3d10
4s2 4p6 4d10
4f14 5s2 5p6
5d10 6s1 or [Xe] 4f14
5d10 6s1 [14, p.7]
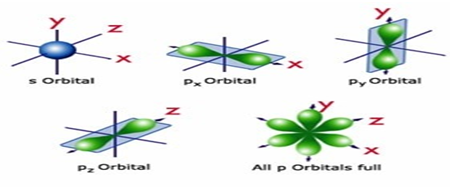
Figure 3.11 Shapes of s, p, and d
orbitals.
The Aufbau Principle. The quantum numbers and the Pauli
exclusion principle define the maximum number of the electrons that can be found
in each of the electron orbits in an atom and also explain how the electrons are
arranged. The aufbau principle (German meaning “to build up” thus also
known as the building-up principle) explains the order in which the electrons
occupy the orbitals. According to this principle the lowest energy orbitals in
an atom are filled before those in the higher energy levels. Each orbital can
accommodate at most two electrons (confirmed by spectroscopic and chemical
analysis). According to additional rule, called the Hund’s rule, if two or more
energetically equivalent orbitals are available (such as orbitals p,
d, f) the electrons spread out before they start to pair. The
reason for this is that because the electrons repel each other and because each
orbital is directed toward a different section in space, the electrons can
depart from each other. The Hund’s rule also says that the unpaired electrons in
degenerate orbitals have the same spin alignment [13, p.
44].
Mendeleev’s Periodic Table. As previously described, the periodic table is a tabular arrangement
of the elements that groups similar elements together. Mendeleev s work
attracted more attention than Meyer’s for two reasons: He left blank spaces in
his table for undiscovered elements, and he corrected some atomic mass values.
The blanks in his table came at atomic masses 44, 68, 72, and 100 for the
elements we now know as scandium, gallium, germanium, and technetium. Two of
the atomic mass values he corrected were those of indium and
uranium.
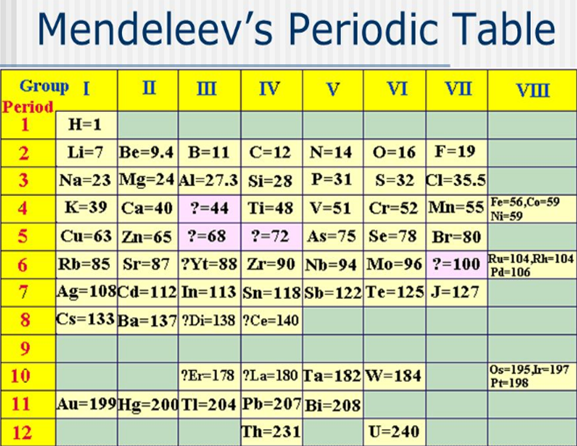
Figure 3.12 Mendeleev’s periodic table
In Mendeleev s table, similar elements fall in vertical groups, and
the properties of the elements change gradually from top to bottom in the group.
As an example, we have seen that the alkali metals (Mendeleev s group I) have
high molar volumes (figure 3.13). They also have low melting points, which
decrease in the order
Li (174 °C) ˃ Na (97.8 °C) ˃ K (63.7 °C) ˃ Rb (38.9 °C) ˃ Cs (28.5
°C)
In their compounds, the alkali metals exhibit the oxidation state +1, forming ionic compounds, such as NaCl, KBr, CsI, Li2O, and so on [3, p.361].
Discovery of New Elements. Three elements predicted by
Mendeleev were discovered shortly after the appearance of his 1871
periodic table (gallium, 1875; scandium, 1879; germanium, 1886).
Table illustrates how closely Mendeleev s predictions for
eka-silicon agree with the observed properties of the element germanium,
discovered in 1886. Often, new ideas in science take hold slowly, but the
success of Mendeleev s predictions stimulated chemists to adopt his table fairly
quickly.
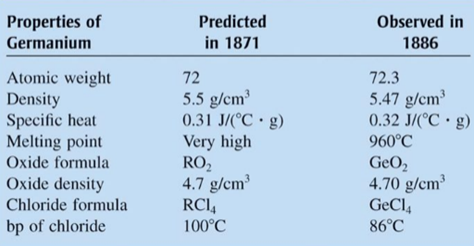
Figure 3.13 Comparison of the properties of Germanium as Predicted by
Mendeleev and as Actually observed
One group of elements that Mendeleev did not anticipate was the noble
gases. He left no blanks for them. William Ramsay, their discoverer, proposed
placing them in a separate group of the table. Because argon, the first noble
gas discovered (1894), had an atomic mass greater than that of chlorine and
comparable to that of potassium, Ramsay placed the new group, which he called
group 0, between the halogen elements (group VII) and the alkali metals (group
I) [3, p.362].
The Periodic Table and Properties of the Elements. By the
mid-19th century, several chemists had discovered that when the elements are arranged by
atomic mass they demonstrate periodic behavior. In 1869, while writing a book on
chemistry, Russian scientist Dmitri Mendeleev (1834–1907) realized this
periodicity of the elements and he arranged them into a table that is today
called the periodic table of elements. The table, as first published, was a
simple observation of regularities in nature; the principles that defined this
periodicity were not understood. Mendeleev’s table contained gaps due to the
fact that some of the elements were yet unknown. In addition, when he arranged
the elements in the table he noticed that the weights of several elements were
wrong.
In the modern periodic table, the elements are grouped in order of
increasing atomic number and arranged in rows (figure 3.14). Elements with
similar physical and chemical properties appear in the same columns. A new row
starts whenever the last (outer) electron shell in each energy level (principal
quantum number) is completely filled. Properties of an element are discussed in
terms of their chemical or physical characteristics. Chemical properties are
often observed through a chemical reaction, while physical properties are
observed by examining a pure element.
The chemical properties of an element are determined by the
distribution of electrons around the nucleus, particularly the outer, or
valence, electrons. Since a chemical reaction does not affect the atomic
nucleus, the atomic number remains unchanged. For example, Li, Na, K, Rb and Cs
behave chemically similarly because each of these elements has only one electron
in its outer orbit. The elements of the last column (He, Ne, Ar, Kr, Xe and Rn)
have filled inner shells and all except helium have eight electrons in their
outermost shells. Because their electron shells are completely filled, these
elements cannot interact chemically and are therefore referred to as the inert,
or noble, gases.
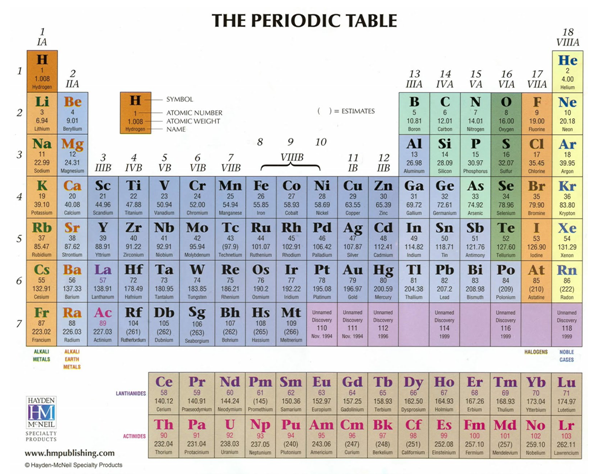
Figure 3.14 The periodic table of elements
Each horizontal row in the periodic table of elements is called a
period. The first period contains only two elements, hydrogen and helium. The
second and third periods each contain eight elements, while the fourth and fifth
periods contain 18 elements each. The sixth period contains 32 elements that are
usually arranged such that elements from Z = 58 to 71 are detached from
main table and placed below it. The seventh and last period is also divided into
two rows, one of which, from Z = 90 to 103, is placed below the second
set of elements from the sixth period. The vertical columns are called groups
and are numbered from left to right. The first column, Group 1, contains
elements that have a closed shell plus a single s electron in the next
higher shell. The elements in Group 2 have a closed shell plus two s
electrons in the next shell. Groups 3–18 are characterized by the elements that
have filled, or almost filled, p levels. Group 18 is also called Group 0
and contains the noble gases. The columns in the interior of the periodic table
contain the transition elements in which the electrons are present in the d
energy level. These elements begin in the fourth period because the first
d level (3d) is in the fourth shell. The sixth and the seventh
shells contain 4f and 5f levels and are called lanthanides, or
rare earth elements, and actinides, respectively. The elements are also grouped
according to their physical properties; for instance, they are grouped into
metals, non-metals, and metalloids. Elements with very similar chemical
properties are referred to as families; examples include the halogens, the inert
gases, and the alkali metals. The following sections only focus on those atomic
properties that are closely related to the principles of nuclear engineering
[13, p. 45].
Block classification of the periodic table. Based on the
composition of electron orbitals, hydrogen, helium and Group 1 elements are
classified as s-block elements, Group 13 through Group 18 elements
p-block elements, Group 3 through Group 12 elements d-block
elements, and lanthanoid and actinoid elements f-block elements.
s-Block, p-block, and Group 12 elements are called main group
elements and d-block elements other than Group 12 and f-block elements
are called transition elements. The characteristic properties of the elements
that belong to these four blocks are described in Chapter 4 and
thereafter. Incidentally, periodic tables that denote the groups of
s-block and p-block elements with Roman numerals (I, II, ... ,
VIII) are still used, but they will be unified into the IUPAC system in the near
future. Since inorganic chemistry covers the chemistry of all the elements, it is important to
understand the features of each element through reference to the periodic table
[14, p. 11].
Bonding states of elements. Organic compounds are molecular
compounds that contain mainly carbon and hydrogen atoms. Since inorganic
chemistry deals with all compounds other than organic ones, the scope of
inorganic chemistry is vast. Consequently, we have to study the syntheses,
structures, bonding, reactions, and physical properties of elements, molecular
compounds, and solid-state compounds of 103 elements. In recent years, the
structures of crystalline compounds have been determined comparatively easily by
use of single crystal X-ray structural analysis, and by through the use of
automatic diffractometers. This progress has resulted in rapid development of
new areas of inorganic chemistry that were previously inaccessible. Research on
higher dimensional compounds, such as multinuclear complexes, cluster compounds,
and solid-state inorganic compounds in which many metal atoms and ligands are
bonded in a complex manner, is becoming much easier. In this section, research
areas in inorganic chemistry will be surveyed on the basis of the classification
of the bonding modes of inorganic materials.
(a) Element.
Elementary substances
exist in various forms. For example, helium and other rare gas elements exist as
single-atom molecules; hydrogen, oxygen, and nitrogen as two-atom molecules;
carbon, phosphorus, and sulfur as several solid allotropes; and sodium, gold,
etc. as bulk metals. A simple substance of a metallic element is usually
called bulk metal, and the word “metal” may be used to mean a bulk metal and
“metal atom or metal ion” define the state where every particle is discrete.
Although elementary substances appear simple because they consist of only one
kind of element, they are rarely produced in pure forms in nature. Even after
the discovery of new elements, their isolation often presents difficulties. For
example, since the manufacture of ultra high purity silicon is becoming very
important in science and technology, many new urification processes have been
developed in recent years.
(b) Molecular
compounds. Inorganic
compounds of nonmetallic elements, such as gaseous carbon dioxide
CO2, liquid sulfuric acid H2SO4, or solid
phosphorus pentoxide P2O5, satisfy the valence
requirements of the component atoms and form discrete molecules which are not
bonded together. The compounds of main group metals such as liquid tin
tetrachloride SnCl4 and
solid aluminum trichloride
AlCl3 have definite molecular weights and do not form infinite
polymers.
Most of the
molecular compounds of transition metals are metal complexes and organometallic
compounds in which ligands are coordinated to metals. These molecular compounds
include not only mononuclear complexes with a metal center but also multinuclear
complexes containing several metals, or cluster complexes having metal-metal
bonds. The number of new compounds with a variety of bonding and structure types
is increasing very rapidly, and they represent a major field of study in today’s
inorganic chemistry.
(c) Solid-state
compounds. Although
solid-state inorganic compounds are huge molecules, it is preferable to define
them as being composed of an infinite sequence of 1-dimensional (chain),
2-dimensional (layer), or 3-dimensional arrays of elements and as having no
definite molecular weight. The component elements of an inorganic solid bond
together by means of ionic, covalent, or metallic bonds to form a solid
structure. An ionic bond is one between electronically positive (alkali metals
etc.) and negative elements (halogen etc.), and a covalent bond
forms between elements with close electronegativities. However, in many
compounds there is contribution from both ionic and covalent bonds.
The
first step in the identification of a compound is to know its elemental
composition. Unlike an organic compound, it is sometimes difficult to decide the
empirical formula of a solid-state inorganic compound from elemental analyses
and to determine its structure by combining information from spectra. Compounds
with similar compositions may have different coordination numbers around a
central element and different structural dimensions. For example, in the case of
binary (consisting of two kinds of elements) metal iodides, gold iodide, AuI,
has a chain-like structure, copper iodide, CuI, a zinc blende type structure,
sodium iodide, NaI, has a sodium chloride structure, and cesium iodide, CsI, has
a cesium chloride structure, and the metal atoms are bonded to 2, 4, 6 or 8
iodine atoms, respectively. The minimum repeat unit of a solid structure is
called a unit lattice and is the most fundamental information in the structural chemistry of crystals. X-ray
and neutron diffraction are the most powerful experimental methods for
determining a crystal structure, and the bonds between atoms can only be
elucidated by using them. Polymorphism is the phenomenon in which
different kinds of crystals of a solid-state compound are obtained in which the
atomic arrangements are not the same. Changes between different polymorphous
phases with variations in temperature and/or pressure, or phase
transitions, are an interesting and important problem in solid-state chemistry
or physics.
We should keep in mind that in solid-state inorganic chemistry the
elemental composition of a compound are not necessarily integers. There are
extensive groups of compounds, called nonstoichiometric compounds, in which the
ratios of elements are non-integers, and these non-stoichiometric compounds
characteristically display conductivity, magnetism, catalytic nature, color, and
other unique solid-state properties. Therefore, even if an inorganic compound
exhibits non-integral stoichiometry, unlike an organic compound, the compound
may be a thermodynamically stable, orthodox compound. This kind of compound is
called a non-stoichiometric compound or Berthollide compound, whereas a
stoichiometric compound is referred to as a Daltonide compound. The law of
constant composition has enjoyed so much success that there is a tendency to
neglect non-stoichiometric compounds. We should point out that groups of
compounds in which there are slight and continuous changes of the composition of
elements are not rare [14, p. 12].