Laboratory work
The preparation of acids
Purpose of the work:
To acquaint with the ways and properties of substances belonging to
various classes of inorganic compounds, to write equations of reactions. Ensure
genetic bonding of acids.
Materials:
·
Sodium chloride
·
Concentrated sulfuric acid
·
Test tube
·
Stopper
·
Distillated water
·
Methylorange
Procedure:
1.
In a clean test tube (№1), pour 2-3 ml of distilled water and add 2
drops of methylorange.
2.
Put a small amount of sodium chloride in the tube №2 with a gas-seal
tape, add 10-15 drops of concentrated sulfuric acid, quickly cover with a sieve
and insert the tube end into the water in the tube №1.
3.
What kind of phenomenon do you recognize? Write the reactions
equations and name them. Write the observed changes in the form of a
summary.
Preparation of bases
Purpose of the work:
To acquaint with the ways and properties of substances belonging to
various classes of inorganic compounds, to write equations of reactions. Ensure
genetic bonding of bases.
Materials:
- Sodium metal stored under mineral oil
- Distilled water
- Phenolphthalein
- Tweezers
- knife
- 250mL beaker
Procedure:
- Add a few drops of phenolphthalein indicator to the water in the
beaker. (Optional)
- You may wish to place the beaker on an overhead projector screen,
which will give you a way to show the reaction to students from a
distance.
- While wearing gloves, use a dry spatula to remove a very small
chunk (0.1 cm3) of sodium metal from the piece stored in the oil.
Return the unused sodium to the oil and seal the container. You can use tongs
or tweezers to dry the small piece of metal on a paper towel. You may wish to
allow the students to examine the cut surface of the sodium. Instruct the
students that they can look at the sample but must not touch the sodium
metal.
- Drop the piece of sodium into the water. Immediately stand back. As
water dissociates into H+ and OH-, hydrogen gas will be evolved. The increasing concentration of
OH- ions in the solution will raise its pH and cause the liquid to
turn pink.
- After the sodium has reacted completely, you can flush it with
water and rinse it down the drain. Continue to wear eye protection when
disposing of the reaction, just in case a bit of unreacted sodium
remained.
Questions:
1.
What is the acidity, the basis and the salt in terms of electrolytic
dissociation theory? Give examples and give definitions.
2.
Describe how to get the basics and give examples. Write the reactions
equations.
3.
Complete these equations:
а) H3PO4 + КОН → ? b) H3PO4 + 2КОН →
?
c)H3PO4
+ 3 КОН→ ?
Name the salts and
calculate the equivalence mass of phosphoric acid in each
reaction.
4.
Which of these bases can not form salts? Zn (OH)2, Cr (OH)3, NaОН ,
Pb (OH)2, AI(OH)3, KOH, NH4OH
5.
Write the following reaction equations that carry out these
changes:
Fe2O3 → FeСI3 → Fe(ОН)3 →
Fe(NO3)3 →
Fe2O3
The preparation of oxygen
Purpose of the work:
In this experiment, we will produce oxygen by the decomposition of
hydrogen peroxide, using manganese dioxide as a catalyst. The gas will be collected using the
water displacement method, and you will investigate one of the oxygen’s
properties.
Materials:
·
3% Hydrogen Peroxide
·
2 hole stopper
·
Manganese dioxide
·
rubber tubing
·
scoopula
·
Tap water
·
matches/sparker
·
balance
·
Wooden splints
·
ring stand
·
bent glass tube
·
1 large test tube
·
glycerin
·
Erlenmeyer flask
·
2-3 small test tubes
·
massing paper
·
thistle tube
·
250mL beaker
·
clamp
Procedure:
1.
Mass out 1.00 g of manganese dioxide on a piece of massing
paper. Pour into to 250mL
Erlenmeyer flask.
2.
Measure 25mL of water with a graduated cylinder and pour into the
flask.
3.
Clamp the Erlenmeyer flask to the ring stand.
4.
Insert the thistle tube and bent glass rod into the two-holed rubber
stopper using glycerin as a lubricant. Your hands must be kept close together
when inserting the glass rod. Otherwise it may break and go through your
hand.
5.
Insert the two-hole into the Erlenmeyer flask, so the bottom thistle
tube is below the water line.
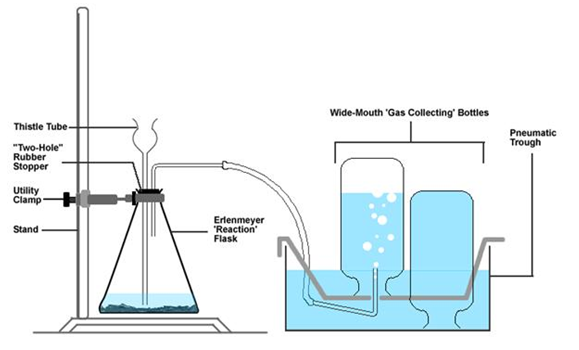
6.
Fill the collection bath with water and submerge the 3 gas collection
test tubes in the bath. Attach the hose from the bath to the glass tube in the
2-holed rubber stopper.
7.
Measure about 200mL of 3% hydrogen peroxide, and slowly pour this
into the thistle tube. Be careful not to make it overflow. If at any time the
rate of the reaction in the Erlenmeyer flask appears to slow down, add another
10-mL portion of H2O2.
8.
After about 80 bubbles have escaped from the tube, take a second test
tube, fill it with water and place it over the rubber collection tube. Allow the water to be displaced by the
oxygen until the tube is full of the oxygen gas. Fill as many test tubes as you
can.
9.
Next, take a wood splint and set it on fire. Then, blow out the flame, but make sure
the splint remains glowing. Now,
pick up the test tube of oxygen gas, turn it horizontally and quickly place the
glowing splint to the mouth of the test tube.
10. Take a small ball of steel wool with crucible tongs, light it on fire
and insert it into the test tube (pointed away from every one) while its
lit.
11. Light a small strip of magnesium, using the same method in step
9.
Questions:
1.
What is a chemical catalyst?
2.
What is the purpose of waiting for 80 bubbles to escape from the gas
collection tube before collecting the oxygen gas?
3.
What did you observe when you placed the glowing splint in the
presence of the oxygen gas?
4.
What difference did you observe between the reactions of Magnesium in
the air versus the reaction in pure oxygen?
5.
What difference did you observe between the reactions of steel wool
in the air versus the reaction in pure oxygen?
6.
Write the 2 balanced chemical reactions of the questions 4 and 5.
(You may use the internet or text books.)
The preparation of salts
Purpose of the work:
Carry out an exchange reaction between copper (II) oxide and sulfuric
acid; to obtain crystals of copper sulfate; to establish in practice the ability
to correctly handle laboratory equipment; To develop independence, creative
activity, as well as logical thinking, the ability to analyze, compare to draw
conclusions.
Materials:
·
spirit lamp
·
matches
·
probe holder
·
test tubes
·
Copper (II) oxide
·
Sulfuric acid
Procedure:
1.
In a dry tube place one glass (pharmacy) spatula of copper (II)
oxide.
2.
Pour 10 drops of sulfuric acid solution to it.
3.
Heat the mixture for 10-15 seconds without boiling (for this purpose,
periodically remove the spirit lamp or remove the test tube from the
flame).
4.
Allow the resulting hot mixture to settle.
5.
Gently drain the solution into a clean test
tube.
6.
Place a drop of hot solution on a slide. (Copper sulfate crystals
precipitate from the solution of copper (II) sulphate.)
7.
The resulting crystals are examined under a
microscope.
8.
Draw crystals of copper sulfate.