Teaching of atomic and molecular structure
Modern definitions of concepts: element, atom, molecule, mole. The
law of conservation of energy and its importance in
chemistry
Low of conservation of Mass. Various apparent changes in weight are observed as a result of
chemical reactions. For example, if we allow a wax candle to burn, we observe
that the candle loses weight. However, when an iron nail rusts in moist air, it
gains weight. It might therefore seem that, in chemical reactions, weight may be
either gained or lost.
However, on closer observation, this is seen not to be the case. If
chemical reactions are carried out in a closed vessel, so that nothing is added
and nothing escapes, we discover that the overall weight of the vessel and its
contents remains the same after the experiment as before. When the nail rusts
under these conditions and the metal takes up a gas (oxygen) from air, the
weight lost by the air is the same as that gained by the nail. Similarly, there
is no overall change in weight when the candle burns, because the gases formed
as it combines with oxygen (carbon dioxide and water vapor) cannot escape from
the vessel; the weight of these gases is exactly as the weight lost by the air
and the candle [5, p.23].
Quite generally, in all chemical processes, the total weight of the
reactants is unchanged. Since the weight G of a given mass m is a function of the gravitational
field at that point: G=mg
(weight = mass ∙ gravitation al acceleration, Appendix II), it is
reasonable to ignore this gravitational contribution (which is a constant) and
to use total mass in place of total weight.
This gives the law of
conservation of mass: In all chemical
processes, the total mass of the reactants remains unchanged.
The full significance of this was recognized in 1774 by the French
chemist Antoine Laurent Lavoisier (1743-1794). Important contributions to the
formulation of this law had been made earlier by M. W. Lomonosov in 1765, and by
J. B. van Helmont in 1620, a century and a half before.
To test this fundamental law of conservation of mass experimentally,
all possible sources of error must be rigorously considered. Such extremely
careful and precise experiments were carried out in 1908 by the German physical
chemist Hans Landolt (1831 - 1910), and in 1909 by the Hungarian physical
chemist Loránd v. Eötvös (1848-1919).
Landolt placed the two reacting solutions in the vessel shown in
figure 1.1, with one solution on each side. He closed the vessel by heating and
sealing the glass of the filling tubes, and then weighed it as precisely as
possible.
The vessel was next
inverted, so that the two solutions mixed and reacted. After the reaction was
complete, the vessel was again weighed very precisely. The total mass of the
solutions used in these experiments was around 300 g; in no case did the change
in mass exceed the limits of error, found earlier in blank experiments to be
0.00003 g.
Therefore, any changes in mass must have been less than
0.00003/300=1/10000000. i.e. 10-5 % of the mass of the reactants.
Eötvös was able to reduce the limits of error by another factor of ten. Within
these limits, therefore, the law of conservation of mass is clearly
obeyed.

However, if it were possible to increase the precision of the mass
determination beyond that attained by Landolt and v. Eötvös, it would be found
that the law of conservation of mass is no longer strictly applicable. This is
because in nearly all chemical reactions, not only is matter converted, but
energy is also either released or absorbed. As we know, any quantity of energy
E is equivalent to the mass m, given by the Einstein equation
(1):
E = mc2
(1),
if m = mass in kg, c = velocity of light in
ms-1, E = energy in
J
Thus if a chemical reaction
releases 500000J in heat energy, this corresponds to a loss in mass of E/c2=500000/(2.997925 ∙
108)2 = 5.5632 ∙ 10-12kg. In order to detect
this loss, the measurement would have to be exactly reproducible within
10-6mg, which is 4 powers of 10 smaller than the precision of 3 ∙
10-2mg achieved by Landolt. Neither Landolt nor von Eötvös could
therefore have detected the mass changes predicted for chemical reactions by the
Einstein equation, especially since the heat released the mass in their
reactions was much less than the value of 500000J assumed
above.
Although it is still
impossible, even with the most sensitive balances and experimental methods now
available, to detect the minute mass changes associated with energy changes in
ordinary reactions, the mass-energy law is clearly valid when considering the
much larger energies involved in transmutation of elements. In such process, the
law of conservation of mass and conservation of energy change as a term in the
equation. The separate laws of conservation of mass and conservation of energy
must therefore be combined into a single law of mass plus
energy.
The law of conservation of mass refers to the total mass in chemical
reactions. When we study the combining mass ratios in chemical reactions other
interesting relationships emerge. The law concerning these theses ratios are
called “stoichiometric laws” (“stoicheion”
is the Greek word for “basic material” , “metron” is Greek for
“measure”).
Stoichiometric Laws. Law of Constant Proportions. Water is a chemical compound; it can be decomposed into gaseous hydrogen
and gaseous oxygen by adding energy (e.g. thermal or electrical energy):
Water + Energy → Hydrogen + Oxygen.
Hydrogen, like oxygen (see above), cannot be separated into simpler
substances by ordinary physical and chemical methods, and is therefore an
element.
Water can be decomposed into its elemental compounds in a Hofmann apparatus (figure 1.1). This consists of three tubes,
connected at the bottom. Water is poured in through the funnel on the middle
tube until the two outer tubes are filled up to the stopcocks, which are then
closed. In the lower part of each of the two outer tubes, there is a small piece
of platinum foil with a platinum connecting wire. As soon as the platinum wires
are connected to a direct current source of sufficiently high voltage, small
bubbles begin to form on the platinum plates (electrodes). The water is being
electrically decomposed into hydrogen and oxygen; this process is known as
electrolysis. Hydrogen, a gas which burns but does not support combustion, forms
at the electrode connected to the negative terminal of the voltage source
(cathode), while oxygen, a gas which supports combustion but will not burn,
forms on the positive electrode (anode). Since pure water conducts electric
current very poorly, a little sulfuric acid is added to the water to increase
its conductivity. If the masses of the oxygen and hydrogen produced are now
determined, it is found that the mass ratio oxygen: hydrogen is always 7.936:1.
This ratio is independent of the method used to split the water, and does not
depend on the experimental conditions, such as the amount of water decomposed,
temperature, pressure, current density, etc.
The same conclusion is reached when water is synthesized from
hydrogen and oxygen:
Hydrogen + Oxygen → Water + Energy
This may be carried out, for example, in the left-hand, calibrated
side of the mercury-filled apparatus sketched in Fig. 15; this is called an eudiometer tube, and was invented by A.
Volta. A mixture of hydrogen and oxygen is initiated by a small electric spark.
The hydrogen and oxygen combine explosively, releasing as heat the same amount
of energy as that needed to decompose water. The water is deposited as extremely
fine droplets on the inner wall of the tube. Here again, the combining mass
ratio of oxygen = 7.936:1. If one of the gases is present in excess of this mass
ratio, that same amount of excess gas remains unchanged at the end of the
reaction.
Similar results are found for other chemical reactions. If, for
example, hydrogen chloride (known as
hydrochloric acid in aqueous
solution) is decomposed into its constituent gaseous elements, hydrogen and
chloride, the mass ratio of chlorine: hydrogen is always 35.175:1. Ammonia always contains nitrogen and
hydrogen in the ratio 4.632:1. Methane (also known as mine gas or marsh
gas) consists of carbon and hydrogen, always in the ratio 2.979:1. The same
ratios are found when hydrogen chloride, ammonia and methane are synthesized
from hydrogen and chlorine, hydrogen and nitrogen, or hydrogen and
carbon:
Hydrogen chloride + energy ↔ hydrogen +
chlorine
Ammonia + energy ↔ hydrogen + nitrogen
Methane + energy ↔ hydrogen + carbon
These and many other experiments illustrate a general law, called the
law of constant proportions: The mass ratio of two elements which combine
to form a particular chemical compound is constant. The law was formulated
in 1799 by the French chemist Joseph Louis Proust
(1754-1826).
Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas
Law. By the end of this section, you will be able
to:
·
Identify the mathematical relationships between the various
properties of gases
·
Use the ideal gas law, and related gas laws, to compute the values of
various gas properties under specified conditions
During the
seventeenth and especially eighteenth centuries, driven both by a desire to
understand nature and a quest to make balloons in which they could fly, a number
of scientists established the relationships between the macroscopic physical
properties of gases, that is, pressure, volume, temperature, and amount of gas.
Although their measurements were not precise by today’s standards, they were
able to determine the mathematical relationships between pairs of these
variables (e.g., pressure and temperature, pressure and volume) that hold for an
ideal gas—a hypothetical construct that real gases approximate under
certain conditions. Eventually, these individual laws were combined into a
single equation—the ideal gas law—that relates gas quantities for gases and is quite accurate for low
pressures and moderate temperatures. We will consider the key developments in
individual relationships (for pedagogical reasons not quite in historical
order), then put them together in the ideal gas law [2, p.
477].
Pressure and Temperature: Amontons’s Law. Imagine filling a rigid container attached to a pressure gauge with
gas and then sealing the container so that no gas may escape. If the
container is cooled, the gas inside likewise gets colder and its pressure is
observed to decrease. Since the container is rigid and tightly sealed,
both the volume and number of moles of gas remain constant. If we heat
the sphere, the gas inside gets hotter (figure 1.2) and the pressure
increases.
This relationship between temperature and pressure is observed for
any sample of gas confined to a constant volume. An example of experimental
pressure-temperature data is shown for a sample of air under these conditions in
figure 1.2. We find that temperature and pressure are linearly related, and if
the temperature is on the kelvin scale, then P and T are directly proportional (again, when volume and moles of gas are held constant); if the temperature on the kelvin scale increases by a certain
factor, the gas pressure increases by the same factor.
Guillaume Amontons was the first to empirically establish the
relationship between the pressure and the temperature of a gas (~1700), and
Joseph Louis Gay-Lussac determined the relationship more precisely (~1800).
Because of this, the P-T relationship for gases is known as either Amontons’s law or Gay-Lussac’s law. Under either name, it states that the pressure of a given amount of gas is directly proportional to its
temperature on the kelvin scale when the volume is held constant. Mathematically, this can be written (2):
P ∝ T or P = constant × T or P = k × T (2)
where ∝ means “is proportional to,” and k is a proportionality constant that depends on the identity, amount,
and volume of the gas.
For a confined, constant volume of gas, the ratio P/T is therefore constant (i.e.,P/T = k). If the gas is initially
in “Condition 1” (with P = P1 and T = T1) (3), and then changes to “Condition 2” (with P = P2 and T = T2) (4), we have that
P1/T1 = k and P2/T2 = k
(3),
which reduces to P1/T1 = P2/T2
(4).
This equation is useful for pressure-temperature calculations for a confined gas at constant volume. Note that temperatures must be on the kelvin scale for any gas law calculations (0 on the kelvin scale and the lowest possible temperature is called absolute zero). (Also note that there are at least three ways we can describe how the pressure of a gas changes as its temperature changes: We can use a table of values, a graph, or a mathematical equation.)
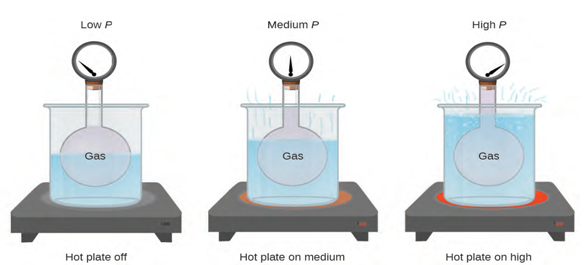
Figure 1.2 The effect of temperature on gas pressure: When the hot plate is off,
the pressure of the gas in the sphere is relatively low. As the gas is heated,
the pressure of the gas in the sphere increases [2, p.478]
Volume and Temperature: Charles’s Law. If we fill a balloon with air and seal it, the balloon contains a
specific amount of air at atmospheric pressure, let’s say 1 atm. If we
put the balloon in a refrigerator, the gas inside gets cold and the balloon
shrinks (although both the amount of gas and its pressure remain
constant). If we make the balloon very cold, it will shrink a great deal, and
it expands again when it warms up.
These examples of the effect of temperature on the volume of a given
amount of a confined gas at constant pressure are true in general: The volume
increases as the temperature increases, and decreases as the temperature
decreases. Volume-temperature data for a 1-mole sample of methane gas at 1 atm
are listed and graphed in figure 1.3.
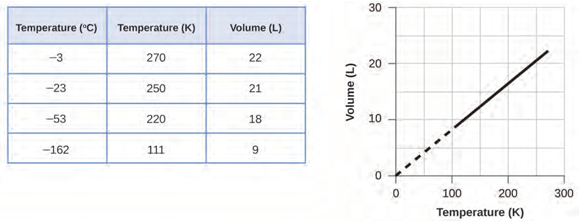
Figure 1.3 The volume and temperature are linearly related for 1 mole of methane
gas at a constant pressure of 1 atm. If the temperature is in kelvin, volume and
temperature are directly proportional. The line stops at 111 K because methane
liquefies at this temperature; when extrapolated, it intersects the graph’s
origin, representing a temperature of absolute zero.
The relationship between the volume and temperature of a given amount
of gas at constant pressure is known as Charles’s law in recognition of the
French scientist and balloon flight pioneer Jacques Alexandre Cesar Charles.
Charles’s law states that the volume of a given amount of gas is directly proportional to its
temperature on the Kelvin scale when the pressure is held constant.
Mathematically, this can be written as (5):
V α
T or V = constant·T or V = k·T or V1 /T1 = V2 /T2 (5)
with k being a proportionality constant that depends on the amount and
pressure of the gas. For a confined, constant pressure gas sample,
V/T is constant (i.e., the ratio = k), and as seen with the V-T relationship, this leads to another form of Charles’s
law: V1 /T1 = V2 /T2 [2, p.480].
Volume and Pressure: Boyle’s Law. If we partially fill an airtight syringe with air, the syringe
contains a specific amount of air at constant temperature, say 25 °C. If
we slowly push in the plunger while keeping temperature constant, the gas in the
syringe is compressed into a smaller volume and its pressure increases;
if we pull out the plunger, the volume increases and the pressure decreases.
This example of the effect of volume on the pressure of a given amount of a
confined gas is true in general. Decreasing the volume of a contained gas
will increase its pressure, and increasing its volume will decrease its
pressure. In fact, if the volume increases by a certain factor, the
pressure decreases by the same factor, and vice versa. Volume-pressure
data for an air sample at room temperature are graphed in figure
1.4.
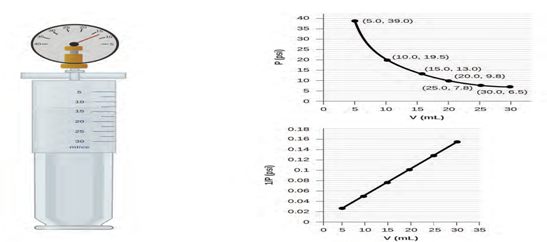
Figure 1.4 When a gas occupies a smaller volume, it exerts a higher pressure;
when it occupies a larger volume, it exerts a lower pressure (assuming the
amount of gas and the temperature do not change). Since P and V are inversely proportional, a graph of 1/P vs. V is linear.
Unlike the P-T and V-T relationships, pressure and volume are not directly proportional to
each other. Instead, P and V exhibit inverse proportionality: Increasing the pressure results in a
decrease of the volume of the gas. Mathematically this can be written (6):
P α 1/V or P = k·1/V or P·V = k or P1 V1 = P2 V2 (6)
with k being a constant. Graphically, this relationship is shown by the
straight line that results when plotting the inverse of the pressure
(
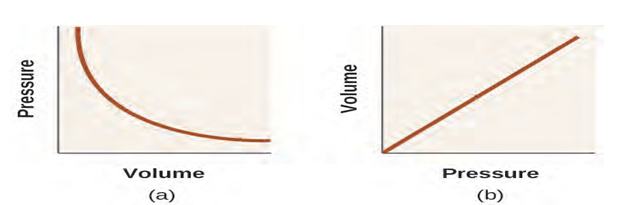
Figure 1.5 The relationship between pressure and volume is inversely
proportional. (a) The graph of P vs. V is a parabola, whereas (b) the graph of (1/P) vs. V is linear.
The relationship between the volume and pressure of a given amount of
gas at constant temperature was first published by the English natural
philosopher Robert Boyle over 300 years ago. It is summarized in the statement
now known as Boyle’s law: The volume of a given amount of gas held at constant temperature is
inversely proportional to the pressure under which it is
measured [2, p. 482].
Moles of Gas and Volume: Avogadro’s Law. The Italian scientist Amedeo Avogadro advanced a hypothesis in 1811
to account for the behavior of gases, stating that equal volumes of all
gases, measured under the same conditions of temperature and pressure, contain
the same number of molecules. Over time, this relationship was supported
by many experimental observations as expressed by Avogadro’s law: For a confined gas, the volume (V) and number of moles (n) are
directly proportional if the pressure and temperature both remain constant.
In equation form, this is written as (7):
V ∝ n or V = k × n or V1/n1 = V2/n2
(7)
Mathematical relationships can also be determined for the other
variable pairs, such as P versus n, and n versus T [2, p. 486].
The Ideal Gas Law. To this point, four separate laws have been discussed that relate
pressure, volume, temperature, and the number of moles of the
gas:
·
Boyle’s law: PV = constant at constant T and n
·
Amontons’s law:P/T = constant at constant V and n
·
Charles’s law: V/T= constant at constant P and n
·
Avogadro’s law: V/n = constant at constant P and T
Combining these four laws yields the ideal gas law, a relation between the pressure, volume, temperature, and number of
moles of a gas (8):
PV = nRT (8)
where P is the pressure of a gas, V is its volume, n is the number of moles of the gas, T is its temperature on the kelvin scale, and R is a constant called the ideal gas constant or the universal gas constant. The units used to express pressure,
volume, and temperature will determine the proper form of the gas constant as
required by dimensional analysis, the most commonly encountered values being
0.08206 L atm mol–1 K–1 and 8.314 kPa L mol–1
K–1.
Gases whose properties of P, V, and T are accurately described by the ideal gas law (or the other gas laws)
are said to exhibit ideal behavior or to approximate the traits of an ideal gas. An ideal gas is a hypothetical construct that may be used along
with kinetic molecular theory to effectively explain the gas laws as will be described in a later
module of this chapter. Although all the calculations presented in this module
assume ideal behavior, this assumption is only reasonable for gases under
conditions of relatively low pressure and high temperature. In the final module
of this chapter, a modified gas law will be introduced that accounts for the
non-ideal behavior observed for many gases at relatively high pressures and low
temperatures.
The ideal gas equation contains five terms, the gas constant
R and the variable properties P, V, n, and T. Specifying any four of these terms will permit use of the ideal gas
law to calculate the fifth term as demonstrated in the following example
exercises [2, p.487].
Standard Conditions of Temperature and Pressure. We have seen that the volume of a given quantity of gas and the
number of molecules (moles) in a given volume of gas vary with changes in
pressure and temperature. Chemists sometimes make comparisons against a
standard temperature and pressure (STP) for reporting properties of gases: 273.15 K and 1 atm (101.325 kPa).
At STP, an ideal gas has a volume of about 22.4 L—this is referred to as
the standard molar volume (figure
1.6).
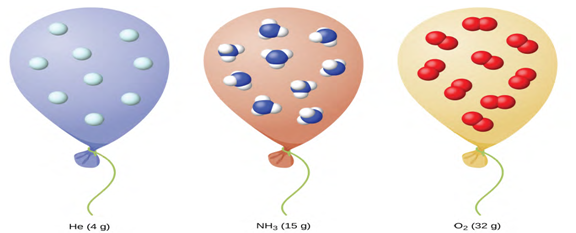
Figure 1.6 Since the number of moles in a given volume of gas varies with
pressure and temperature changes, chemists use standard temperature and pressure
(273.15 K and 1 atm or 101.325 kPa) to report properties of gases [2, p.
490]
Law of Multiple Proportions. Two elements often combine with each other in more than one ratio,
forming several different compounds. For example, nitrogen and oxygen combine to
form five different compounds.
If the mass ratios of these elements in their various compounds are
compared, it is seen that they are not arbitrary, independent numbers. Instead
there is a simple relationship between them. The amounts of oxygen which combine
with a given amount of nitrogen in these compounds are in the ratios
1:2:3:4:5.
Compound 1: Mass ratio oxygen: nitrogen = 0.571:1 = (1 ∙ 7.936) : (3 ∙ 4.632),
Compound 2: Mass ratio oxygen: nitrogen = 1.142:1 = (2 ∙ 7.936) : (3 ∙ 4.632),
Compound 3: Mass ratio oxygen: nitrogen = 1.7131:1 = (3 ∙ 7.936) : (3 ∙ 4.632),
Compound 4: Mass ratio oxygen: nitrogen = 2.284:1 = (4 ∙ 7.936) : (3 ∙ 4.632),
Compound 5: Mass ratio oxygen: nitrogen = 2.855:1 = (5 ∙ 7.936) : (3 ∙ 4.632).
Similar relationships are seen in many other cases: if two elements A
and B each form one compound with a third element C, then the mass ratio in a
compound consisting of A and B is never totally unrelated, but is a combination
of two numbers which are multiples of the mass rations in the first two
compounds. This law, which was recognized in essence in 1791 by the German
chemist Jeremias Benjamin Richter (1762-1807), is called the law of equivalent proportions: Elements always combine with one another to
form chemical compounds in a ratio of definite combining masses (“equivalent
masses”) or integral multiples of these masses. This law goes beyond the
scope of both the preceding laws, and includes them as
well.
Stoichiometry of Gaseous Substances, Mixtures, and
Reactions. By the end of this section, you will be able
to:
·
Use the ideal gas law to compute gas densities and molar
masses
·
Perform stoichiometric calculations involving gaseous
substances
·
State Dalton’s law of partial pressures and use it in calculations
involving gaseous mixtures
The study of the chemical behavior of gases was part of the basis of
perhaps the most fundamental chemical revolution in history. French nobleman
Antoine Lavoisier, widely regarded as the “father of modern chemistry,” changed
chemistry from a qualitative to a quantitative science through his work with
gases. He discovered the law of conservation of matter, discovered the role of
oxygen in combustion reactions, determined the composition of air, explained
respiration in terms of chemical reactions, and more. He was a casualty of the
French Revolution, guillotined in 1794. Of his death, mathematician and
astronomer Joseph-Louis Lagrange said, “It took the mob only a moment to remove
his head; a century will not suffice to reproduce it.”
As described in an earlier chapter of this text, we can turn to
chemical stoichiometry for answers to many of the questions that ask “How much?”
We can answer the question with masses of substances or volumes of solutions.
However, we can also answer this question another way: with volumes of gases. We
can use the ideal gas equation to relate the pressure, volume, temperature, and
number of moles of a gas. Here we will combine the ideal gas equation with other
equations to find gas density and molar mass. We will deal with mixtures of
different gases, and calculate amounts of substances in reactions involving
gases. This section will not introduce any new material or ideas, but will
provide examples of applications and ways to integrate concepts we have already
discussed [2, p. 491].
Density of a Gas. Recall that the density of a gas is its mass to volume ratio,
ρ
= mV. Therefore, if we can determine the mass of some volume of a gas, we will get its density. The density of an
unknown gas can used to determine its molar mass and thereby assist in its identification. The ideal gas law,
PV = nRT, provides us with a means of deriving such a mathematical formula to relate the density of a gas to its volume in
the proof.
Derivation of a Density Formula from the Ideal Gas Law.
Use PV = nRT to derive a formula for the density of gas (9) in
g/L.
Step 1. PV = nRT
Step 2. Rearrange to get (mol/L): nv = P/RT
Step 3. Multiply each side of the equation by the molar mass, ℳ. When moles are multiplied by ℳ in g/mol, g are obtained:
(ℳ)(n/V) = (P/RT)(ℳ)
Step 4. m/V = ρ
= P ℳ/RT (9)
We must specify
both the temperature and the pressure of a gas when calculating its density
because the number of moles of a gas (and thus the mass of the gas) in a liter
changes with temperature or pressure. Gas densities are often reported at STP
[2, p.491].
Molar Mass of a Gas. Another useful application of the ideal gas law involves the
determination of molar mass. By definition, the molar mass of a substance
is the ratio of its mass in grams, m, to its amount in moles, n (10):
ℳ = grams of substance/moles of substance = m/n
The ideal gas equation can be rearranged to isolate n (11):
n = PV/RT (11)
and then combined with the molar mass equation to yield (12):
ℳ = mRT/PV (12)
This equation can be used to derive the molar mass of a gas from
measurements of its pressure, volume, temperature, and mass [2, p.
492].
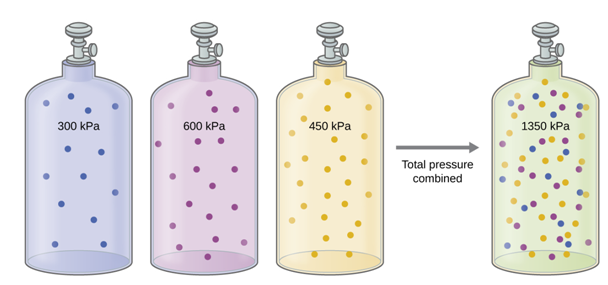
The Pressure of a Mixture of Gases: Dalton’s Law. Unless they chemically react with each other, the individual gases in
a mixture of gases do not affect each other’s pressure. Each individual gas in a
mixture exerts the same pressure that it would exert if it were present alone in
the container (Figure 1.7). The
pressure exerted by each individual gas in a mixture is called its partial pressure. This observation is summarized by Dalton’s law of partial pressures:
The total pressure of a mixture of ideal gases is equal to the sum of
the partial pressures of the component gases (13):
PTotal = PA + PB + PC + ... = Σi Pi (13)
In the equation PTotal is the total pressure of a mixture of gases, PA is the partial pressure of gas A; PB is the partial pressure of gas B; PC is the partial pressure of gas C; and so on.
The partial pressure of gas A is related to the total pressure of the
gas mixture via its mole fraction (X), a unit of concentration defined as the number of moles of a
component of a solution divided by the total number of moles of all components
(14):
PA = XA × PTotal
where XA = nA/nTotal
(14)
where PA, XA, and nA are the partial pressure, mole fraction, and number of moles of gas
A, respectively, and nTotal is the number of moles of all components in the
mixture.
Collection of Gases over Water. A simple way to collect gases that do not react with water is to
capture them in a bottle that has been filled with water and inverted into a
dish filled with water. The pressure of the gas inside the bottle can be made
equal to the air pressure
outside by raising or lowering the bottle. When the water level is the same both
inside and outside the bottle
(figure 1.8), the
pressure of the gas is equal to the atmospheric pressure, which can be measured
with a barometer.
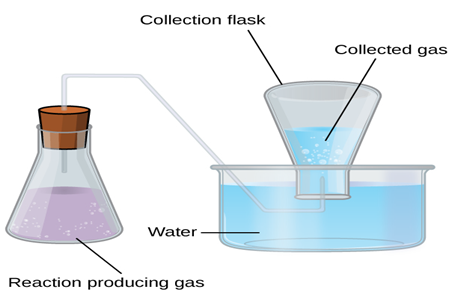
Figure 1.8 When a reaction produces a gas that is collected above water, the
trapped gas is a mixture of the gas produced by the reaction and water vapor. If
the collection flask is appropriately positioned to equalize the water levels
both within and outside the flask, the pressure of the trapped gas mixture will
equal the atmospheric pressure outside the flask (see the earlier discussion of
manometers).
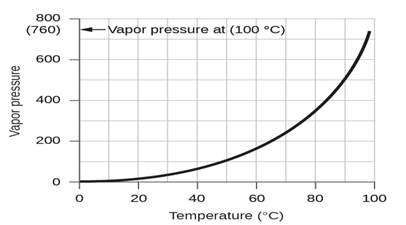
Figure 1.9 This graph shows the vapor pressure of water at sea level as a
function of temperature.
However, there is another factor we must consider when we measure the
pressure of the gas by this method. Water evaporates and there is always gaseous
water (water vapor) above a sample of liquid water. As a gas is collected over
water, it becomes saturated with water vapor and the total pressure of the
mixture equals the partial pressure of the gas plus the partial pressure of the
water vapor. The pressure of the pure gas is therefore equal to the total
pressure minus the pressure of the water vapor—this is referred to as the “dry”
gas pressure, that is, the pressure of the gas only, without water vapor. The
vapor pressure of water, which is the pressure exerted by water vapor in equilibrium with
liquid water in a closed container, depends on the temperature(figure 1.9); more
detailed information on the temperature dependence of water vapor can be found ,
and vapor pressure will be discussed in more detail in the next chapter on
liquids [2, p. 496].
Chemical Stoichiometry and Gases. Chemical stoichiometry describes the quantitative relationships
between reactants and products in chemical reactions. We have previously
measured quantities of reactants and products using masses for solids and
volumes in conjunction with the molarity for solutions; now we can also
use gas volumes to indicate quantities. If we know the volume, pressure,
and temperature of a gas, we can use the ideal gas equation to calculate how
many moles of the gas are present. If we know how many moles of a gas are
involved, we can calculate the volume of a gas at any temperature and
pressure [1, p.498].
Dalton’s Atomic Hypothesis. A simple illuminating interpretation of all the laws discussed above
was given by J. Dalton; he drew on earlier ideas of J. Jungius, proposed even
before the stoichiometric laws had been experimentally confirmed Dalton’s atom hypothesis, published in 1808,
stated that chemical elements are not infinitely disable, but consist of tiny,
chemically indivisible particles, the so-called “atoms” (from the Greek “atomos”
= indivisible). All the atoms of a given element A have the same mass, while the
masses of the atoms of two different elements A and B differ by a constant
amount. (Due to the existence of isotopes (q. v.) of an element, “average mass”
must now be substituted for “mass”).
If element A combines with element B to give a chemical compound,
this implies that a atoms of A
combine with b atoms of B to give the
smallest particles AaBb of the chemical compound;
a and b are integers. Examples of possible
reactions are:
A + B → AB or
2A + B → A2B or
A + 2B → AB2 or
2A + 3B → A2B3, etc.
Since the atoms forming the compounds gave characteristic masses, all
the stoichiometric laws discussed so far easily explained.
The law of conservation of
mass is a natural consequence of the atom hypothesis because there is no
conversion of matter in a chemical reaction, but only a combination or
rearrangement of atoms, so that the total mass of the chemical system naturally
remains unchanged. According to the atom hypothesis, the experimentally observed
constant and multiple mass ratios, predicted by the laws of the constant and multiple
proportions, reflect the ratios of the atomic masses of the elements or
integral multiples of those masses. Similarly, the ratios of the combining
masses observed experimentally and expressed by the law of equivalent proportions are none
other than the ratios of the atomic masses or their integral
multiples.
The mass ratios of the atoms of the individual elements (relative
atomic masses) cannot be unequivocally determined from the mass ratios observed
when chemical compounds are formed from the elements. This is because the
combining ratios of atoms are not initially known. In the formation of water,
for example,, if 1 hydrogen atom combined with 1 oxygen atom, the experimentally
observed mass ratio of hydrogen:oxygen = 1:7.936 would indicate that an oxygen
atom is 2:1 or 11:2, the atomic mass of oxygen is then either twice or half as
large, namely either 7.936 ∙ 2 = 15.872 or 7.936 : 2 = 3.968 (relative to
hydrogen with atomic mass 1).
To determine relative atomic masses, we must therefore know the
numerical ratios in which the atoms combine to give chemical compounds. It will
now be shown that, for gaseous reactants, these ratios can be found simply by
determining the ratios of combining volumes of the
elements.
Atoms and the Atomic Theory. We begin this chapter with a brief
survey of early chemical discoveries, culminating in Dalton’s atomic theory.
This is followed by a description of the physical evidence leading to the modern
picture of the nuclear atom, in which protons and neutrons are combined
into a nucleus with electrons in space surrounding the nucleus. We will also
introduce the periodic table as the primary means of organizing elements into
groups with similar properties. Finally, we will introduce the concept of the
mole and the Avogadro constant, which are the principal tools for counting atoms
and molecules and measuring amounts of substances. We will use these tools
throughout the text.
Early Chemical Discoveries and the Atomic Theory. Chemistry has been practiced for a very long time, even if its
practitioners were much more interested in its applications than in its
underlying principles. The blast furnace for extracting iron from iron ore
appeared as early as A.D. 1300, and such important chemicals as sulfuric acid
(oil of vitriol), nitric acid, and sodium sulfate (Glauber’s salt) were all well
known and used several hundred years ago. Before the end of the eighteenth
century, the principal gases of the atmosphere nitrogen and oxygen had been
isolated, and natural laws had been proposed describing the physical behavior of
gases. Yet chemistry cannot be said to have entered the modern age until the
process of combustion was explained. In this section, we explore the direct link
between the explanation of combustion and Dalton s atomic theory [3, p.
35].
Law of Constant Composition. In 1799, Joseph Proust (1754 1826) reported, One hundred pounds of
copper, dissolved in sulfuric or nitric acids and precipitated by the carbonates
of soda or potash, invariably gives 180 pounds of green carbonate. * This and
similar observations became the basis of the law of constant composition, or the
law of definite proportions:
All samples of a compound have the same composition the same
proportions by mass of the constituent elements.
To see how the law of constant composition works, consider the
compound water. Water is made up of two atoms of hydrogen (H) for every atom of
oxygen (O), a fact that can be represented symbolically by a chemical
formula, the familiar H2O.
The substance Proust produced is actually a more complex substance
called basic copper carbonate. Proust s results were valid because, like
all compounds, basic copper carbonate has a constant composition [3, p.
36].
Law of Conservation of Mass. The first of these Fundamental Laws to be discovered was the Law of
Conservation of Mass.
The total mass of material
present after a chemical reaction is the same as before the
reaction.
This Law was discovered by
Antoine Lavoisier in about 1789. In a turn about of the Scientific Method,
Lavoisier had always assumed this Law was true, and sought out experiments which
would verify his assumptions. As a result of numerous combustion experiments
conducted on systems in closed containers, so as to retain any gases present,
Lavoisier was able to unambiguously verify his assumptions and formally state
the Law of Conservation of Mass.
For an example, consider our combustion reactions of elemental
Carbon. If the mass of the gasses are accounted for, it is
found:
Carbon
+ Oxygen → Carbonic acid
Before Rxn:
1.00g
2.66g
= 3.66g
After Rxn:
0.00g
0.00g
3.66g
= 3.66g
Carbon + Oxygen → Carbonic Oxide
Before Rxn:
1.00g
1.66g
= 2.66g
After Rxn
0.00g
0.00g
2.66g
= 2.66g
Of course, these results require that each reactant be present in
perfectly balanced amounts, such that the full quantity of each is consumed
completely during the reaction. If this is not the case, some of the reagent in
excess will remain at the conclusion of the reaction. However, the Law of
Conservation of Mass will still apply.
Hydrogen + Oxygen → Water
Before Rxn:
2.00g
10.00g
= 12.00g
After Rxn:
0.74g
0.00g
11.26g
= 12.00g
From this example, we see a total of 12.00g of material is present
both before and after the chemical reaction occurs, with some of the hydrogen
reagent remaining as excess. Further, we can also note that oxygen is the
Limiting Reagent in carrying out this reaction; it limits the production of
water. If more oxygen were present, a greater amount of water would be
produced.
Finally, once this Law is accepted, it can be used to predict the
amount of an "unseen" reactant consumed or produced without direct measurement.
For instance, when iron burns in the air, its mass is seen to
increase:
Iron + Oxygen → Iron Oxide
Before Rxn:
5.00g ?g
After Rxn:
0.00g 0.00g
7.15g
From these results we can calculate the mass of oxygen needed to
carry-out the complete combustion of 5.00g of iron:
mass Oxygen = 7.15g - 5.00g = 2.15g
Finally, it must be noted the Law of Conservation of Mass, though a
Fundamental Law of Chemistry, is not a fundamental law of nature. When an energy
difference occurs during a reaction, minute amounts of mass are either gained or
lost. Mass is either converted to energy or energy is converted to mass. The
energy-mass equivalence was first postulated by Einstein in his famous formula;
E = mc2. While these mass differences are not detectable by the
chemist, they are important in nuclear reactions.
Dalton’s Atomic Theory. From 1803 to 1808, John
Dalton, an English schoolteacher, used the two fundamental laws of chemical
combination just described as the basis of an atomic theory. His theory involved
three assumptions:
1.
Each chemical element is composed of minute, indivisible particles
called atoms. Atoms can be neither created nor destroyed during a chemical
change.
2.
All atoms of an element are alike in mass (weight) and other
properties, but the atoms of one element are different from those of all other
elements.
3.
In each of their compounds, different elements combine in a simple
numerical ratio, for example, one atom of A to one of B (AB), or one atom of A
to two of
If atoms of an element are indestructible (assumption 1), then the
same atoms must be present after a chemical reaction as before. The total
mass remains unchanged. Dalton s theory explains the law of conservation of
mass. If all atoms of an element are alike in mass (assumption 2) and if atoms
unite in fixed numerical ratios (assumption 3), the percent composition
of a compound must have a unique value, regardless of the origin of the sample
analyzed. Dalton’s theory also explains the law of constant composition. Like
all good theories, Dalton s atomic theory led to a prediction the law
of multiple proportions. B (AB2).
If two elements form more than a single compound, the masses of one
element combined with a fixed mass of the second are in the ratio of small whole numbers.
To illustrate, consider two oxides of carbon (an oxide is a
combination of an element with oxygen). In one oxide, 1.000 g of carbon is
combined with 1.333 g of oxygen, and in the other, with 2.667 g of oxygen. We
see that the second oxide is richer in oxygen; in fact, it contains twice as
much oxygen as the first, 2.667 g>1.333 g = 2.00.
We now know that the first oxide corresponds to the formula CO and
the second, CO2 The characteristic relative masses of the atoms of
the various elements became known as atomic weights, and throughout the
nineteenth century, chemists worked at establishing reliable values of relative
atomic weights. Mostly, however, chemists directed their attention to
discovering new elements, synthesizing new compounds, developing techniques for
analyzing materials, and in general, building up a vast body of chemical
knowledge. Efforts to unravel the structure of the atom became the focus of
physicists, as we see in the next several sections [3, p.37].
Electrons and Other Discoveries in Atomic Physics. Fortunately, we can acquire a qualitative understanding of atomic
structure without having to retrace all the discoveries that preceded atomic
physics. We do, however, need a few key ideas about the interrelated phenomena
of electricity and magnetism, which we briefly discuss here. Electricity and
magnetism were used in the experiments that led to the current theory
of atomic structure.
Certain objects display a property called electric charge, which can
be either Positive (+) or negative (-). Positive and negative charges attract
each other, while two positive or two negative charges repel each other. As we
learn in this section, all objects of matter are made up of charged particles.
An object having equal numbers of positively and negatively charged particles
carries no net charge and is electrically neutral. If the number of positive
charges exceeds the number of negative charges, the object has a net positive
charge. If negative charges exceed positive charges, the object has a net
negative charge. Sometimes when one substance is rubbed against another, as in
combing hair, net electric charges build up on the objects, implying that
rubbing separates some positive and negative charges. Moreover, when a
stationary (static) positive charge builds up in one place, a negative charge of
equal size appears somewhere else; charge is balanced [3, p. 38].
The Discovery of Electrons. CRT, the abbreviation for cathode-ray tube, was once a familiar
acronym. Before liquid crystal display (LCD) was available, the CRT was the
heart of computer monitors and TV sets. The first cathode-ray tube was made by
Michael Faraday (1791 1867) about 150 years ago. When he passed electricity
through glass tubes from which most of the air had been evacuated, Faraday
discovered cathode rays, a type of radiation emitted by the negative
terminal, or cathode. The radiation crossed the evacuated tube to the
positive terminal, or anode. Later scientists found that cathode rays
travel in straight lines and have properties that are independent of the cathode
material (that is, whether it is iron, platinum, and so on).. The cathode rays
produced in the CRT are invisible, and they can be detected only by the light
emitted by materials that they strike. These materials, called phosphors,
are painted on the end of the CRT so that the path of the cathode rays can be
revealed. (Fluorescence is the term used to describe the emission of
light by a phosphor when it is struck by energetic radiation.) Another
significant observation about cathode rays is that they are deflected by
electric and magnetic fields in the manner expected for negatively charged
particles.
In 1897, by the method outlined, J. J. Thomson (1856 1940)
established the ratio of mass (m) to electric charge (e) for
cathode rays, that is, Also, Thomson concluded that cathode rays are negatively
charged fundamental particles of matter found in all atoms. (The
properties of cathode rays are independent of the composition of the
cathode.) Cathode rays subsequently became known as electrons, a term
first proposed by George Stoney in 1874.
Robert Millikan (1868 1953) determined the electronic charge e
through a series of oil-drop experiments (1906 1914). The currently accepted
value of the electronic charge e, expressed in coulombs to five
significant figures, is - 1.6022 × 10-19 C. By combining this value with an accurate
value of the mass-to-charge ratio for an electron, we find that the mass of an
electron is 9.1094 × 10-28 g.
Once the electron was seen to be a fundamental particle of matter
found in all atoms, atomic physicists began to speculate on how these particles
were incorporated into atoms. The commonly accepted model was that proposed by
J. J. Thomson. Thomson thought that the positive charge necessary to
counterbalance the negative charges of electrons in a neutral atom was in the
form of a nebulous cloud. Electrons, he suggested, floated in a diffuse cloud of
positive charge (rather like a lump of gelatin with electron fruit embedded in
it).
This model became known as the plum-pudding model because of its
similarity to a popular English dessert. The plum-pudding model is illustrated
for a neutral atom and for atomic species, called ions, which carry a net
charge [3, p. 39].
X-Rays and Radioactivity. Cathode-ray research had many important spin-offs. In particular, two
natural phenomena of immense theoretical and practical significance were
discovered in the course of other investigations.
In 1895, Wilhelm Roentgen (1845 1923) noticed that when cathode-ray
tubes were operating, certain materials outside the tubes glowed or
fluoresced. He showed that this fluorescence was caused by radiation emitted by
the cathode-ray tubes. Because of the unknown nature of this radiation, Roentgen
coined the term X-ray. We now recognize the X-ray as a form of
high-energy electromagnetic radiation, which is discussed.
Antoine Henri Becquerel (1852 1908) associated X-rays with
fluorescence and wondered if naturally fluorescent materials produce X-rays. To
test this idea, he wrapped a photographic plate with black paper, placed a coin
on the paper, covered the coin with a uranium-containing fluorescent material,
and exposed the entire assembly to sunlight. When he developed the film, a clear
image of the coin could be seen. The fluorescent material had emitted radiation
(presumably X-rays) that penetrated the paper and exposed the film. On one
occasion, because the sky was overcast, Becquerel placed the experimental
assembly inside a desk drawer for a few days while waiting for the weather to
clear. On resuming the experiment, Becquerel decided to replace the original
photographic film, expecting that it may have become slightly exposed. He
developed the original film and found that instead of the expected feeble image,
there was a very sharp one. The film had become strongly exposed because the
uranium-containing material had emitted radiation continuously, even when it was
not fluorescing. Becquerel had discovered radioactivity.
Ernest Rutherford (1871 1937) identified two types of radiation from
radioactive materials, alpha and beta Alpha particles carry two
fundamental units of positive charge and have essentially the same mass as
helium atoms. In fact, alpha particles are identical to He2+ ions.
Beta particles are negatively charged particles produced by changes
occurring within the nuclei of radioactive atoms and have the same properties as
electrons. A third form of radiation, which is not affected by electric or
magnetic fields, was discovered in 1900 by Paul Villard. This radiation, called
gamma rays, is not made up of particles; it is electromagnetic radiation of
extremely high penetrating power. These three forms of radioactivity are
illustrated.
By the early 1900s, additional radioactive elements were discovered,
principally by Marie and Pierre Curie. Rutherford and Frederick Soddy made
another profound finding: The chemical properties of a radioactive element
change as it undergoes radioactive decay. This observation suggests that
radioactivity involves fundamental changes at the subatomic level in
radioactive decay, one element is changed into another, a process known as
transmutation [3, p. 41].
The Nuclear Atom. In 1909, Rutherford, with his assistant Hans Geiger, began a line of
research using a particles as probes
to study the inner structure of atoms. Based on Thomson s plum-pudding model,
Rutherford expected that most particles in a beam of a particles would pass through thin
sections of matter largely undeflected, but that some a particles would be slightly scattered
or deflected as they encountered electrons. By studying these scattering
patterns, he hoped to deduce something about the distribution of electrons in
atoms.
Alpha particles were detected by the flashes of light they produced
when they struck a zinc sulfide screen mounted on the end of a telescope. When
Geiger and Ernst Marsden, a student, bombarded very thin foils of gold with
particles, they observed the following:
·
The majority of particles penetrated the foil
undeflected.
·
Some a particles
experienced slight deflections.
·
A few (about 1 in every 20,000) suffered rather serious deflections
as they penetrated the foil.
·
A similar number did not pass through the foil at all, but bounced
back in the direction from which they had come.
The large-angle scattering greatly puzzled Rutherford. As he
commented some years later, this observation was about as credible as if you had
fired a 15-inch shell at a piece of tissue paper and it came back and hit you.
By 1911, though, Rutherford had an explanation. He based his explanation on a
model of the atom known as the nuclear atom and having these
features:
1.
Most of the mass and all of the positive charge of an atom are
centered in a very small region called the nucleus. The remainder of the
atom is mostly empty space.
2.
The magnitude of the positive charge is different for different atoms
and is approximately one-half the atomic weight of the
element.
3.
There are as many electrons outside the nucleus as there are units of
positive charge on the nucleus. The atom as a whole is electrically neutral [3,
p. 42].
Discovery of Protons and Neutrons. Rutherford s nuclear atom suggested the existence of positively
charged fundamental a particles of
matter in the nuclei of atoms. Rutherford himself discovered these particles,
called protons, in 1919 in
studies involving the scattering of particles by nitrogen atoms in air. The
protons were freed as a result of collisions between a particles and the nuclei of nitrogen
atoms. At about this same time, Rutherford predicted the existence in the
nucleus of electrically neutral fundamental particles. In 1932, James Chadwick
showed that a newly discovered penetrating radiation consisted of beams of
neutral particles. These particles, called neutrons, originated from the nuclei
of atoms. Thus, it has been only for about the past 100 years that we have had
the atomic model suggested [3, p. 43].
Properties of Protons, Neutrons, and Electrons. The number of protons in a given atom is called the atomic number, or the proton number, Z. The number of
electrons in the atom is also equal to Z because the atom is electrically
neutral. The total number of protons and neutrons in an atom is called the mass number, A. The number of
neutrons, the neutron number,
is A - Z. An
electron carries an atomic unit of negative charge, a proton carries an atomic
unit of positive charge, and a neutron is electrically neutral. The atomic mass unit (described
more fully on page 46) is defined as exactly 1/12 of the mass of the atom known
as carbon-12 (read as carbon twelve). An atomic mass unit is abbreviated and
denoted by the symbol u.
The three subatomic particles considered in this section are the only
ones involved in the phenomena of interest to us in this text. You should be
aware, however, that a study of matter at its most fundamental level must
consider many additional subatomic particles. The electron is believed to be a
truly fundamental particle. However, modern particle physics now considers the
neutron and proton to be composed of other, more fundamental particles
[3, p. 44].
The Concept of the Mole and the Avogadro Constant. At the same temperature and pressure, equal volumes of different
gases contain the same number of particles.
Starting with Dalton, chemists have recognized the importance of
relative numbers of atoms, as in the statement that two hydrogen atoms
and one oxygen atom combine to form one molecule of water. Yet it
is physically impossible to count every atom in a macroscopic sample of matter.
Instead, some other measurement must be employed, which requires a relationship
between the measured quantity, usually mass, and some known, but uncountable,
number of atoms. Consider a practical example of mass substituting for a desired
number of items. Suppose you want to nail down new floorboards on the deck of a
mountain cabin, and you have calculated how many nails you will need. If you
have an idea of how many nails there are in a pound, then you can buy
the nails by the pound.
The SI quantity that describes an amount of substance by relating it
to a number of particles of that substance is called the mole
(abbreviated mol). A mole is the amount of a substance that
contains the same number of elementary entities as there are atoms in exactly 12
g of pure carbon-12. The number of elementary entities (atoms, molecules, and so
on) in a mole is the
Avogadro constant, NA
NA = 6.02214179 * 1023 mol-1
The Avogadro constant consists of a number, 6.02214179 ×
1023, known as Avogadro s number, and a unit mol-1.
The unit mol-1 signifies that the entities being counted are those
present in 1 mole. The value of Avogadro s number is based on both a definition
and a measurement. Amole of carbon-12 is defined to be 12 g. If the mass
of one carbon-12 atom is measured by using a mass spectrometer, the mass
would be about 1.9926 × 10-23 g. The ratio of these two masses
provides an estimate of Avogadro s number. In actual fact, accurate
determinations of Avogadro s number make use of other measurements, not the
measurement of the mass of a single atom of carbon-12.
Often the value of NA is rounded off to 6.022 ×
1023 mol-1 or even to 6.02 × 1023
mol-1. If a substance
contains atoms of only a single isotope, then
1 mol 12C = 6.02214 × 1023 12C atoms
= 12.0000 g
1 mol 16O = 6.02214 × 1023 16O atoms
= 15.9949 g (and so on)
Most elements are composed of mixtures of two or more isotopes so
that the atoms in a sample of the element are not all of the same mass but are
present in their naturally occurring proportions. Thus, in one mole of carbon,
most of the atoms are carbon-12, but some are carbon-13. In one mole of oxygen,
most of the atoms are oxygen-16, but some are oxygen-17 and some are oxygen-18.
As a result,
1 mol of C = 6.02214 × 1023 C atoms = 12.0107
g
1 mol of O = 6.02214 × 1023 O atoms = 15.9994 g, and so
on.
The Avogadro constant was purposely chosen so that the mass of one
mole of carbon-12 atoms -exactly 12 g - would have the same numeric value as the mass of a
single carbon-12 atom - exactly 12 u. As a result, for all other elements the
numeric value of the mass in grams of one mole of atoms and the weigh the
daverage atomic mass in atomic mass units are equal. For example, the weighted
average atomic mass of lithium is 6.941 u and the mass of one mole of lithium
atoms is 6.941 g. Thus, we can easily establish the mass of one mole of atoms,
called the molar mass, M, from a table of atomic masses [3,
p.54].
Thinking About Avogadro’s Number. Avogadro s number (6.02214 ∙ 1023) is an enormously large
number and practically inconceivable in terms of ordinary experience. Suppose we
were counting garden peas instead of atoms. If the typical pea had a
volume of about 0.1cm3, the required pile of peas would cover the
United States to a depth of about 6 km (4 mi). Or imagine that grains of wheat
could be counted at the rate of 100 per minute. A given individual might be able
to count out about 4 billion grains in a lifetime. Even so, if all the people
currently on Earth were to spend their lives counting grains of wheat, they
could not reach Avogadro s number. In fact, if all the people who ever lived on
Earth had spent their lifetimes counting grains of wheat, the total would still
be far less than Avogadro s number. (And Avogadro s number of wheat grains is
far more wheat than has been produced in human history.) Now consider a much
more efficient counting device, a modern personal computer; it is capable of
counting at a rate of about 1 billion units per second. The task of counting out
Avogadro s number would still take about 20 million years!
Avogadro s number is clearly not a useful number for counting
ordinary objects. However, when this inconceivably large number is used to count
inconceivably small objects, such as atoms and molecules, the result is a
quantity of material that is easily within our grasp, essentially a handful [3,
p.56].
Using the Mole Concept in Calculations. Throughout the text, the mole concept will provide conversion factors
for problem-solving situations. With each new situation, we will explore how
the mole concept applies. For now, we will deal with the relationship
between numbers of atoms and the mole. Consider the statement: 1 mol S = 32.065g
S. This allows us to write the conversion factors
1 mol S/6,022×1023 S atoms and 32,065g S/1 mol
S
In calculations requiring the Avogadro constant, students often ask
when to multiply and when to divide by NA. One answer is always to
use the constant in a way that gives the proper cancellation of units. Another
answer is to think in terms of the expected result. In calculating a number of
atoms, we expect the answer to be a very large number and certainly never smaller than one. The
number of moles of atoms, conversely, is generally a number of more modest size
and will often be less than one. In the following examples, we use atomic masses
and the Avogadro constant in calculations to determine the number of atoms
present in a given sample. Atomic masses and the Avogadro constant are known
rather precisely, and students often wonder how many significant figures to
carry in atomic masses or the Avogadro constant when performing calculations.
Here is a useful rule of thumb [3,
p.56].